Difference between revisions of "Tissue Sample Collection"
BredaZimkus (Talk | contribs) m (Reverted edits by BredaZimkus (talk) to last revision by RachaelArenstein) |
BredaZimkus (Talk | contribs) (Undo revision 2445 by BredaZimkus (talk)) |
||
| Line 1: | Line 1: | ||
| − | + | == Statement of Purpose == | |
| + | These links and documents contain information about best practices for tissue sample collection. | ||
== Introduction == | == Introduction == | ||
| + | [[File:Slide24.jpeg|thumb|right|Role of genetic resource collections in the research and conservation of amphibians from Zimkus, Hassapakis, and Houck (2019). Green indicates the storage of tissues in biobanks. Purple indicates procedures associated with Assisted Reproductive Technologies (ARTs) that lead to achieving multiple goals in amphibian research and conservation. Asterisk (*) denotes tissue or methodologies that are not currently used in ARTs but may be possible in the future.]] Numerous methods are currently being used to preserve tissue and likely depend on the specific aim of a scientific study or goals of an institutional or collaborative program (e.g., biobank, multi-institution initiative). Samples may be collected for individual research projects with explicit and relatively short-term goals (e.g., molecular ecology, molecular phylogeny, population genetics). Studies may also be taxonomically or regionally focused, such as rapid biodiversity assessments that use DNA barcoding techniques to identify species surveyed in a specific region. In contrast, biobanking initiatives or collaborative programs involving multiple institutions may have targeted specific species for long-term conservation and/or use in Assisted Reproductive Technologies (ARTs). The ultimate aim of a research study or conservation initiative may dictate the specific tissue types or biomolecules needed to fulfill the project goals. Molecular studies traditionally used DNA as it could be preserved more easily in the field with many methods. Unfortunately, the various methods used for DNA preservation are not equally effective, and DNA may be fragmented or otherwise compromised. Some preparations may allow high-quality Sanger sequencing reads but prevent high-quality gDNA needed to sequence genomes or the high-molecular-weight DNA needed for long-read sequencing and other technologies (e.g., BAC library preparation, optical mapping, 10X Chromium libraries). RNA is increasingly being used in gene expression studies but is preserved using fewer methods and degrades rapidly if not frozen immediately. Researchers should, therefore, consider all preservation options as some may allow them to both fulfill their study goals and aid in current or future research or conservation initiatives. In addition, those Researchers collecting samples need to consider the location of initial preservation and if possible carry out a feasibility study to ensure that the selected preservation method(s) will work given any logistical constraints. | ||
| − | == | + | ==Contributors== |
| − | + | Major editor: [[User:BredaZimkus|Breda Zimkus]]. Original content generated during The American Society of Ichthyologists and Herpetologists (ASIH) Annual Joint Meeting - 2016, during an iDigBio sponsored workshop by the following individuals participating in the "Field to Database" Group of the aforementioned workshop: [[User:BredaZimkus|Breda Zimkus]], Cesar Aguilar, Ben Frable, Meredith Mahoney, Zachary Randall, and David Wernecke. | |
| − | == | + | ==Vial Considerations== |
| + | One area of immense variability is with the number of vial types used in natural history collections <ref name=ZimkusFord2014>Zimkus, B.M. and L.S. Ford. 2014. Genetic resource collections associated with natural history museums: A survey and analysis to establish a benchmark of standards. Pp. 9–44 in DNA Banking for the 21st Century. Proceedings of the U.S. Workshop on DNA Banking (W.L. Applequist and L. Campbell, eds.). William L. Brown Center, St. Louis, Missouri. 187 pp.</ref>. | ||
| + | * Samples stored using LN<sub>2</sub> must have vials that are rated for use with cryogenic temperatures, preferably made of polypropylene with screw-top caps. Glass vials are problematic when frozen because of their fragility when handling and vulnerability to cracking when stressed, and their use with LN<sub>2</sub> is unacceptable because leakage into the vials can lead to the vessels exploding. | ||
| + | * Pop-off lids are not recommended because this vial type can easily open on its own. | ||
| + | * Vials can have threads located internally or externally on the vial opening; both vial types exhibit advantages and disadvantages. | ||
| + | ::- Externally-threaded closures promoteb more sterile conditions because internal threading can allow contaminants to enter if the cap is placed on an unclean surface when removed, but these vials can be susceptible to cracks and loss of air-tightness, which can lead to sample dessication or oxidation (Corthals and DeSalle 2005, Corthals 2006). | ||
| + | ::-Internally-threaded vials might allow increased storage capacity, depending on the vial selected, but users also suggest that material can be trapped within the threads of this vial type (Johnson 1999). | ||
| + | * Some manufacturers suggest that vial caps that incorporate a silicone gasket or O-ring (internally or externally threaded) are ideal for vapor-phase LN<sub>2</sub> freezing because the seal is enhanced; however, there are some concerns: | ||
| + | ::- Care must be taken if the vial cap is over-tightened because the gasket can become distended. | ||
| + | ::- The presence of gaskets or O-ring might improve the initial performance of seals but can be problematic when used with some alcohols (e.g., ethanol) because some gasket material, such as silicone, is vapor permeable. | ||
| + | * When working with liquid-phase nitrogen cold storage, extra caution must be taken because the accidental entrapment of liquefied nitrogen inside the vial leads to pressure build up and, upon removal, rapid vaporization of the liquid can result in leakage or even explosion. | ||
| + | ::- As a precaution, vial manufacturers recommend that samples not be immersed in LN<sub>2</sub> unless they have secondary containment. Heat-sealing vials into flexible polyethylene tubing is recommended for safe storage in the liquid-phase environment. | ||
| − | == | + | ==Labeling and organizing vials== |
| + | * Pre-label vials | ||
| + | ::-Many researchers include specimen tags inside vials, but paper inside vials not ideal because of possible contamination | ||
| + | * Use alcohol-proof pen | ||
| + | * Use scribe to etch identifying information as back-up | ||
| + | * Organizing vials numerically in boxes is preferable to storage in bags in case writing comes off vials | ||
| + | * Tissue samples should be taken as soon after euthanization as possible | ||
| − | [[ | + | ==Tissue Collection Methods== |
| + | Some general recommendations include: | ||
| + | * Ensure that gloves are worn | ||
| + | * Make sure that all instruments and surfaces are sterilized (or use disposable products) between sub-samples | ||
| + | * Collect sample as soon after euthanasia as possible | ||
| + | ::-Pre-labeling vials may increase preparation speed | ||
| + | * Fill two sets of tissue vials in the field: one for current project and one for institutional collection (rather than subsampling one vial later). | ||
| + | * Label vial to indicate unique number and tissue type | ||
| + | ::- Only place one tissue type in each vial | ||
| + | ::- Epigenetic studies require same tissue type for all samples in study (i.e., all muscle) | ||
| + | * Cut/slice up tissue sample, small size allows permeation by preservative (Note: read specific recommendations for preservative provided by manufacturer.) | ||
| + | * Fresh preservative may be needed if in field for extended period of time | ||
| + | * Don’t over-fill vial with tissue, or tissue and preservative (expansion, can interfere with vial threads, etc.) | ||
| + | |||
| + | ==Tissue Preservation Methods== | ||
| + | The methods currently used to preserve genetic material during initial sampling vary widely owing to the sampling location, tissue type, | ||
| + | and intended research use (Dessauer and Hafner 1984, Prendini et al. 2002, Bus´ and Allen 2014). A comprehensive treatise of methods to preserve genetic samples is discussed in Nagy (2010). In a survey of genetic resource collections associated with natural history | ||
| + | museums, samples were found to be initially preserved in a variety of different ways. Zimkus, Hassapakis, and Houck (2018) discuss these most commonly used methods of sample preservation identified for collections biobanking amphibian genetic resources, addressing the advantages and disadvantages for both researchers making the initial collections and biobankers concerned with long-term storage and downstream use. We provide an overview of these methods, as well as the “tissue piecing” method to allows later initiation of cell culture. | ||
| + | [[File:Slide22.jpeg|center|Comparison of most commonly used preservation methods by Zimkus (unpublished).]] | ||
| + | |||
| + | ===Freezing and Flash-freezing=== | ||
| + | * Freezing samples without the use of a preservation agent either by placing them into a laboratory freezer (≈-20 °C) or by | ||
| + | using ice within an insulated container is generally not recommended because temperatures are not low enough to prevent enzymatic activity nor the formation of intracellular ice crystals (Stoycheva et al. 2007; Nagy 2010). | ||
| + | * Those collecting samples may have access to mechanical freezers that can maintain samples at ultracold or cryogenic temperatures (-80 °C to -150 °C), but this generally excludes samples that are collected in the field. | ||
| + | * Dry ice (frozen carbon dioxide; -78.5 °C) stored within insulated containers may allow the collection or transport of samples, but a sublimation rate of approximately 10% or 2–5 kg every 24 hours limits this method to relatively short trips. In addition, since temperatures near the upper end of the ultra-low temperature range, some DNA degradation may occur as a result of weak enzymatic activity (Nagy 2010). | ||
| + | * Cryogenic storage dewars, a specialized type of vacuum flask used to store cryogenic fluids, can be used to flash-freeze samples in LN<sub>2</sub>. | ||
| + | ::- Access to LN<sub>2</sub> increases field sample collection options, including the preservation of tissues useful for cell culture, RNA, and gametes for multiple weeks. | ||
| + | ::- The use of LN2 requires additional precautions as it can cause frostbite, cold burns, and asphyxiation by displacing the oxygen of the surrounding area. | ||
| + | :: -Sample vials can shatter when removed from storage because LN<sub>2</sub> can enter the vials and rapidly expand upon warming, creating a hazard from both flying debris and exposure to the contents. Secondary containment (e.g., polyethylene tubing, tin foil) and protective eyewear is, therefore, recommended. | ||
| + | ::- Cross-contamination has been reported for samples immersed directly in LN<sub>2</sub>, so researchers should consider vial type, secondary containment options, and use of vapor-phase storage when considering flash-freezing methods (Clark 1999). | ||
| + | ::-Freezing without the inclusion of a preservative was once thought to maximize future research potential, but data now suggests that buffered samples or those stored in a cryoprotectant, such as DMSO or glycerol, may perform better after thawing and refreezing (Nagy 2010) | ||
| + | ::-Cryoprotectants partly protect against degradation occurring during temperature changes, such as freeze-thaw cycles. If viable cells are desired (e.g., cell culture, gametes), slow freezing and using a cryoprotectant is required to prevent the formation of ice crystals that lead to fatal cell lysis. | ||
| + | * A dry shipper is an insulated cryogenic flask/container that contains LN<sub>2</sub> absorbed into a porous lining. | ||
| + | ::- Dry shippers are not considered a dangerous product and hence can be used to ship samples by plane if the liquid is fully absorbed and excess poured off. | ||
| + | ::- There are wide variations in dry shippers with regard to size and temperature (static) hold times. | ||
| + | ::- Size ranges include those with space for a dozen vials to others that can accommodate thousands of vials. | ||
| + | ::- Temperature hold times can vary from a few days to multiple weeks, and variations also exist in how well they hold temperature under different environmental and handling conditions. | ||
| + | |||
| + | ===Ethanol=== | ||
| + | * Using ethanol as a preservative has numerous advantages for researchers whose primary goal is to preserve DNA. | ||
| + | * Ethanol is easy to use and able to preserve DNA even in areas with elevated ambient temperatures for long periods of time. | ||
| + | * Ethanol is flammable and considered hazardous. | ||
| + | ::- The transport of non-infectious ethanol-preserved specimens has been allowed since 2011 via International Air Transport Association (IATA) Special Provision A180, making it possible to transport specimens preserved in ethanol. See [[Shipping and Handling of Dangerous Goods]] for more information about proper packing and labeling. | ||
| + | * Ethanol concentration can greatly affect the resulting quality of the samples with 95–96% (190 proof) recommended as optimal. | ||
| + | ::- Concentrations above 96% (including absolute ethanol) are not recommended as they likely contain traces of drying agents (e.g., benzene) that can affect DNA preservation (Ito 1992). | ||
| + | ::- Concentrations of 65–75% (commonly used to preserve whole animals for morphology) are not recommended; Seutin et al. (1991) were unable to recover DNA from bird brain and muscle samples kept in 70% ethanol for six weeks at room temperature, while liver samples yielded significantly degraded DNA. | ||
| + | ::- Nagy (2010) suggested that tissues be cut into small pieces to increase the surface area, using at least 5:1 volumes ethanol, while others suggest higher ratios (Martin 1977). | ||
| + | ::- Although the initial concentration and ratio of ethanol to sample is important, changing the alcohol during the first one to two days of storage is also recommended because samples release water and progressively dilute the preservative (Kilpatrick 2002; Nagy 2010). | ||
| + | ::- Researchers should avoid using distilled alcoholic beverages because they may have alcohol concentrations as low as 35%. | ||
| + | ::- Undiluted rectified spirits or neutral spirits (e.g., Everclear, Crystal Clear, Primasprit, Spirytus) is highly concentrated (95–96%) but should be avoided because it includes denaturing chemicals. | ||
| + | ::- Denatured alcohol (i.e., methylated spirits), widely used for industrial purposes, is made of 70–99% ethanol but contains additives that | ||
| + | make it non-consumable for humans (e.g., methyl ethyl ketone, also known as MEK) and thus should also be avoided (Post et al. 1993; Dillon et al. 1996). | ||
| + | |||
| + | ===DMSO=== | ||
| + | * DMSO is commonly used in aqueous solutions to preserve DNA as it readily permeates tissues and enhances absorption of materials that inhibit nucleases (e.g., EDTA, NaCl). | ||
| + | * DMSO prevents cellular damage from formation of ice crystals, making it an effective cryoprotectant. | ||
| + | * Solutions can be easily made in the laboratory, are associated with only minor health concerns (e.g., skin irritation), and can be shipped without restrictions (Kilpatrick 2002; Nagy 2010). | ||
| + | ::- DMSO solutions are stable at room temperature, non-toxic and non-flammable. | ||
| + | ::- These solutions can be carried on airplanes more easily than ethanol. | ||
| + | * A number of solutions with 20–25% DMSO, 0.25 M disodium-EDTA, and salt to saturation have been shown to be effective (Dawson et al. 1998; Kilpatrick 2002; Seutin et al. 1991). | ||
| + | ::- Kilpatrick (2002) found that a 3:1 DMSO-salt solution provided the best protection from DNA degradation of mammalian liver tissues stored for up to two years when compared to 95% ethanol and lysis buffer. | ||
| + | ::- Nagy (2010) recommended that the ratio between DMSO and sample exceed 5:1 but at the very least be 3:1 for effective preservation. | ||
| + | * One major issue is that these solutions preserve DNA and not RNA at room temperature. | ||
| + | * There have been no long-term studies to test effects on tissue and DNA quality over periods of time relevant to museum collections. | ||
| + | * For those working with archival samples, tissue can become encrusted with salt, making it more difficult to sub-sample. | ||
| + | |||
| + | ===RNA''later''=== | ||
| + | * RNA''later'' is an aqueous, nontoxic tissue storage reagent marketed to preserve RNA: | ||
| + | ::- up to one day at 37 °C | ||
| + | ::- up to one week at 25 °C | ||
| + | ::- up to one month at 4 °C | ||
| + | ::- indefinitely at temperatures of -20 °C or below. | ||
| + | * It is able to stabilize and protect both RNA and DNA at ambient temperature. | ||
| + | * It is not considered hazardous for shipping, making it easy to transport to and from field collection sites. | ||
| + | * According to both the manufacturer and published studies, RNA''later'' has been tested and found to be successful in preserving many animal tissues (e.g., brain, heart, kidney, spleen, liver, testis, skeletal muscle, fat, lung, and thymus; Nagy 2010; Camacho-Sanchez 2013). | ||
| + | ::- This product is not recommended for bone because of the lack of sufficient penetration into the tissue. | ||
| + | ::- Use of RNA''later'' to preserve RNA in blood and plasma have more involved procedures. | ||
| + | * RNA''later'' should only be used with fresh tissue (previously frozen tissues thaw too slowly in RNAlater, preventing the reagent from diffusing into the tissues quickly enough to prevent nucleic acid degradation) and requires that samples be: | ||
| + | ::- cut into small pieces (i.e., less than 0.5 cm in one dimension), | ||
| + | ::- placed in 5–10 volumes of the solution | ||
| + | ::- incubated overnight at 4 °C to allow thorough penetration; however, if ambient temperature is above 25°C, it is suggested that samples are placed on ice for a few hours after being placed in RNAlater before storing at ambient temperature. | ||
| + | * This product is considered expensive, but researchers have devised homemade versions that may be more cost-effective, although their efficacy is yet untested (Nagy 2010). | ||
| + | |||
| + | ===Tissue Piecing Protocol=== | ||
| + | * Viable fibroblast cell lines are one of the most versatile genetic resources and can play an important role in ex situ conservation. | ||
| + | * Cell lines banked in LN<sub>2</sub> can be maintained indefinitely and provide a continual source of genetic material for a wide variety of purposes (Ryder and Onuma 2018). | ||
| + | ::- These cells can be utilized to obtain chromosomes, expanded to generate large quantities of DNA/RNA, and used for SCNT because they are living and dividing (Houck et al. 2017). | ||
| + | ::-Induced pluripotent stem cells (iPSCs) capable of differentiation into multiple cell types have been generated from skin cells of mammals, including humans, mice, and rhesus monkeys, by direct molecular reprogramming (see Houck et al. 2017 for review). | ||
| + | ::- More recently these methods were successfully applied to cryopreserved adult fibroblasts of endangered mammal species, including a primate (''Mandrillus leucophaeus'') and the northern white rhinoceros (''Ceratotherium simum cottoni''; Ben-Nun et al. 2011; Korody et al. 2017). | ||
| + | * Freezing tissue biopsy samples for later initiation of cell culture (i.e., “tissue piecing,” Fig. 3) is described in Houck et al. (1995), Gamble (2014), and Houck et al. (2017, protocol 24.11); herein we summarize this method. | ||
| + | # Tissue is collected in vials containing cell culture media with antibiotics and held at 4 °C (or room temperature if refrigeration is not available); ideally tissues are stored in media for less than three days but can potentially be stored up to 10 days if no contamination occurs. | ||
| + | # Under aseptic conditions tissue is then minced into 1 mm³ fragments and placed in cell culture medium containing 10% DMSO and either transferred to a primed LN2 dry shipper for short-term storage during transport or placed directly into a long-term LN<sub>2</sub> storage. | ||
| + | # Tissue prepared this way and kept in LN<sub>2</sub> can be stored indefinitely and later transported to a lab with experience in tissue culture to establish cell lines. | ||
| + | [[File:Slide29.jpeg|center|Comparison of most commonly used preservation methods by Zimkus (unpublished).]] | ||
Revision as of 18:55, 13 May 2019
Contents
Statement of Purpose
These links and documents contain information about best practices for tissue sample collection.
Introduction
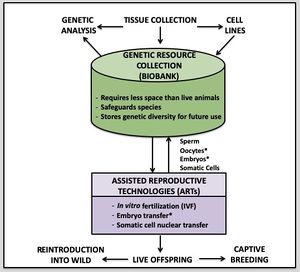
Contributors
Major editor: Breda Zimkus. Original content generated during The American Society of Ichthyologists and Herpetologists (ASIH) Annual Joint Meeting - 2016, during an iDigBio sponsored workshop by the following individuals participating in the "Field to Database" Group of the aforementioned workshop: Breda Zimkus, Cesar Aguilar, Ben Frable, Meredith Mahoney, Zachary Randall, and David Wernecke.
Vial Considerations
One area of immense variability is with the number of vial types used in natural history collections [1].
- Samples stored using LN2 must have vials that are rated for use with cryogenic temperatures, preferably made of polypropylene with screw-top caps. Glass vials are problematic when frozen because of their fragility when handling and vulnerability to cracking when stressed, and their use with LN2 is unacceptable because leakage into the vials can lead to the vessels exploding.
- Pop-off lids are not recommended because this vial type can easily open on its own.
- Vials can have threads located internally or externally on the vial opening; both vial types exhibit advantages and disadvantages.
- - Externally-threaded closures promoteb more sterile conditions because internal threading can allow contaminants to enter if the cap is placed on an unclean surface when removed, but these vials can be susceptible to cracks and loss of air-tightness, which can lead to sample dessication or oxidation (Corthals and DeSalle 2005, Corthals 2006).
- -Internally-threaded vials might allow increased storage capacity, depending on the vial selected, but users also suggest that material can be trapped within the threads of this vial type (Johnson 1999).
- Some manufacturers suggest that vial caps that incorporate a silicone gasket or O-ring (internally or externally threaded) are ideal for vapor-phase LN2 freezing because the seal is enhanced; however, there are some concerns:
- - Care must be taken if the vial cap is over-tightened because the gasket can become distended.
- - The presence of gaskets or O-ring might improve the initial performance of seals but can be problematic when used with some alcohols (e.g., ethanol) because some gasket material, such as silicone, is vapor permeable.
- When working with liquid-phase nitrogen cold storage, extra caution must be taken because the accidental entrapment of liquefied nitrogen inside the vial leads to pressure build up and, upon removal, rapid vaporization of the liquid can result in leakage or even explosion.
- - As a precaution, vial manufacturers recommend that samples not be immersed in LN2 unless they have secondary containment. Heat-sealing vials into flexible polyethylene tubing is recommended for safe storage in the liquid-phase environment.
Labeling and organizing vials
- Pre-label vials
- -Many researchers include specimen tags inside vials, but paper inside vials not ideal because of possible contamination
- Use alcohol-proof pen
- Use scribe to etch identifying information as back-up
- Organizing vials numerically in boxes is preferable to storage in bags in case writing comes off vials
- Tissue samples should be taken as soon after euthanization as possible
Tissue Collection Methods
Some general recommendations include:
- Ensure that gloves are worn
- Make sure that all instruments and surfaces are sterilized (or use disposable products) between sub-samples
- Collect sample as soon after euthanasia as possible
- -Pre-labeling vials may increase preparation speed
- Fill two sets of tissue vials in the field: one for current project and one for institutional collection (rather than subsampling one vial later).
- Label vial to indicate unique number and tissue type
- - Only place one tissue type in each vial
- - Epigenetic studies require same tissue type for all samples in study (i.e., all muscle)
- Cut/slice up tissue sample, small size allows permeation by preservative (Note: read specific recommendations for preservative provided by manufacturer.)
- Fresh preservative may be needed if in field for extended period of time
- Don’t over-fill vial with tissue, or tissue and preservative (expansion, can interfere with vial threads, etc.)
Tissue Preservation Methods
The methods currently used to preserve genetic material during initial sampling vary widely owing to the sampling location, tissue type, and intended research use (Dessauer and Hafner 1984, Prendini et al. 2002, Bus´ and Allen 2014). A comprehensive treatise of methods to preserve genetic samples is discussed in Nagy (2010). In a survey of genetic resource collections associated with natural history museums, samples were found to be initially preserved in a variety of different ways. Zimkus, Hassapakis, and Houck (2018) discuss these most commonly used methods of sample preservation identified for collections biobanking amphibian genetic resources, addressing the advantages and disadvantages for both researchers making the initial collections and biobankers concerned with long-term storage and downstream use. We provide an overview of these methods, as well as the “tissue piecing” method to allows later initiation of cell culture.
Freezing and Flash-freezing
- Freezing samples without the use of a preservation agent either by placing them into a laboratory freezer (≈-20 °C) or by
using ice within an insulated container is generally not recommended because temperatures are not low enough to prevent enzymatic activity nor the formation of intracellular ice crystals (Stoycheva et al. 2007; Nagy 2010).
- Those collecting samples may have access to mechanical freezers that can maintain samples at ultracold or cryogenic temperatures (-80 °C to -150 °C), but this generally excludes samples that are collected in the field.
- Dry ice (frozen carbon dioxide; -78.5 °C) stored within insulated containers may allow the collection or transport of samples, but a sublimation rate of approximately 10% or 2–5 kg every 24 hours limits this method to relatively short trips. In addition, since temperatures near the upper end of the ultra-low temperature range, some DNA degradation may occur as a result of weak enzymatic activity (Nagy 2010).
- Cryogenic storage dewars, a specialized type of vacuum flask used to store cryogenic fluids, can be used to flash-freeze samples in LN2.
- - Access to LN2 increases field sample collection options, including the preservation of tissues useful for cell culture, RNA, and gametes for multiple weeks.
- - The use of LN2 requires additional precautions as it can cause frostbite, cold burns, and asphyxiation by displacing the oxygen of the surrounding area.
- -Sample vials can shatter when removed from storage because LN2 can enter the vials and rapidly expand upon warming, creating a hazard from both flying debris and exposure to the contents. Secondary containment (e.g., polyethylene tubing, tin foil) and protective eyewear is, therefore, recommended.
- - Cross-contamination has been reported for samples immersed directly in LN2, so researchers should consider vial type, secondary containment options, and use of vapor-phase storage when considering flash-freezing methods (Clark 1999).
- -Freezing without the inclusion of a preservative was once thought to maximize future research potential, but data now suggests that buffered samples or those stored in a cryoprotectant, such as DMSO or glycerol, may perform better after thawing and refreezing (Nagy 2010)
- -Cryoprotectants partly protect against degradation occurring during temperature changes, such as freeze-thaw cycles. If viable cells are desired (e.g., cell culture, gametes), slow freezing and using a cryoprotectant is required to prevent the formation of ice crystals that lead to fatal cell lysis.
- A dry shipper is an insulated cryogenic flask/container that contains LN2 absorbed into a porous lining.
- - Dry shippers are not considered a dangerous product and hence can be used to ship samples by plane if the liquid is fully absorbed and excess poured off.
- - There are wide variations in dry shippers with regard to size and temperature (static) hold times.
- - Size ranges include those with space for a dozen vials to others that can accommodate thousands of vials.
- - Temperature hold times can vary from a few days to multiple weeks, and variations also exist in how well they hold temperature under different environmental and handling conditions.
Ethanol
- Using ethanol as a preservative has numerous advantages for researchers whose primary goal is to preserve DNA.
- Ethanol is easy to use and able to preserve DNA even in areas with elevated ambient temperatures for long periods of time.
- Ethanol is flammable and considered hazardous.
- - The transport of non-infectious ethanol-preserved specimens has been allowed since 2011 via International Air Transport Association (IATA) Special Provision A180, making it possible to transport specimens preserved in ethanol. See Shipping and Handling of Dangerous Goods for more information about proper packing and labeling.
- Ethanol concentration can greatly affect the resulting quality of the samples with 95–96% (190 proof) recommended as optimal.
- - Concentrations above 96% (including absolute ethanol) are not recommended as they likely contain traces of drying agents (e.g., benzene) that can affect DNA preservation (Ito 1992).
- - Concentrations of 65–75% (commonly used to preserve whole animals for morphology) are not recommended; Seutin et al. (1991) were unable to recover DNA from bird brain and muscle samples kept in 70% ethanol for six weeks at room temperature, while liver samples yielded significantly degraded DNA.
- - Nagy (2010) suggested that tissues be cut into small pieces to increase the surface area, using at least 5:1 volumes ethanol, while others suggest higher ratios (Martin 1977).
- - Although the initial concentration and ratio of ethanol to sample is important, changing the alcohol during the first one to two days of storage is also recommended because samples release water and progressively dilute the preservative (Kilpatrick 2002; Nagy 2010).
- - Researchers should avoid using distilled alcoholic beverages because they may have alcohol concentrations as low as 35%.
- - Undiluted rectified spirits or neutral spirits (e.g., Everclear, Crystal Clear, Primasprit, Spirytus) is highly concentrated (95–96%) but should be avoided because it includes denaturing chemicals.
- - Denatured alcohol (i.e., methylated spirits), widely used for industrial purposes, is made of 70–99% ethanol but contains additives that
make it non-consumable for humans (e.g., methyl ethyl ketone, also known as MEK) and thus should also be avoided (Post et al. 1993; Dillon et al. 1996).
DMSO
- DMSO is commonly used in aqueous solutions to preserve DNA as it readily permeates tissues and enhances absorption of materials that inhibit nucleases (e.g., EDTA, NaCl).
- DMSO prevents cellular damage from formation of ice crystals, making it an effective cryoprotectant.
- Solutions can be easily made in the laboratory, are associated with only minor health concerns (e.g., skin irritation), and can be shipped without restrictions (Kilpatrick 2002; Nagy 2010).
- - DMSO solutions are stable at room temperature, non-toxic and non-flammable.
- - These solutions can be carried on airplanes more easily than ethanol.
- A number of solutions with 20–25% DMSO, 0.25 M disodium-EDTA, and salt to saturation have been shown to be effective (Dawson et al. 1998; Kilpatrick 2002; Seutin et al. 1991).
- - Kilpatrick (2002) found that a 3:1 DMSO-salt solution provided the best protection from DNA degradation of mammalian liver tissues stored for up to two years when compared to 95% ethanol and lysis buffer.
- - Nagy (2010) recommended that the ratio between DMSO and sample exceed 5:1 but at the very least be 3:1 for effective preservation.
- One major issue is that these solutions preserve DNA and not RNA at room temperature.
- There have been no long-term studies to test effects on tissue and DNA quality over periods of time relevant to museum collections.
- For those working with archival samples, tissue can become encrusted with salt, making it more difficult to sub-sample.
RNAlater
- RNAlater is an aqueous, nontoxic tissue storage reagent marketed to preserve RNA:
- - up to one day at 37 °C
- - up to one week at 25 °C
- - up to one month at 4 °C
- - indefinitely at temperatures of -20 °C or below.
- It is able to stabilize and protect both RNA and DNA at ambient temperature.
- It is not considered hazardous for shipping, making it easy to transport to and from field collection sites.
- According to both the manufacturer and published studies, RNAlater has been tested and found to be successful in preserving many animal tissues (e.g., brain, heart, kidney, spleen, liver, testis, skeletal muscle, fat, lung, and thymus; Nagy 2010; Camacho-Sanchez 2013).
- - This product is not recommended for bone because of the lack of sufficient penetration into the tissue.
- - Use of RNAlater to preserve RNA in blood and plasma have more involved procedures.
- RNAlater should only be used with fresh tissue (previously frozen tissues thaw too slowly in RNAlater, preventing the reagent from diffusing into the tissues quickly enough to prevent nucleic acid degradation) and requires that samples be:
- - cut into small pieces (i.e., less than 0.5 cm in one dimension),
- - placed in 5–10 volumes of the solution
- - incubated overnight at 4 °C to allow thorough penetration; however, if ambient temperature is above 25°C, it is suggested that samples are placed on ice for a few hours after being placed in RNAlater before storing at ambient temperature.
- This product is considered expensive, but researchers have devised homemade versions that may be more cost-effective, although their efficacy is yet untested (Nagy 2010).
Tissue Piecing Protocol
- Viable fibroblast cell lines are one of the most versatile genetic resources and can play an important role in ex situ conservation.
- Cell lines banked in LN2 can be maintained indefinitely and provide a continual source of genetic material for a wide variety of purposes (Ryder and Onuma 2018).
- - These cells can be utilized to obtain chromosomes, expanded to generate large quantities of DNA/RNA, and used for SCNT because they are living and dividing (Houck et al. 2017).
- -Induced pluripotent stem cells (iPSCs) capable of differentiation into multiple cell types have been generated from skin cells of mammals, including humans, mice, and rhesus monkeys, by direct molecular reprogramming (see Houck et al. 2017 for review).
- - More recently these methods were successfully applied to cryopreserved adult fibroblasts of endangered mammal species, including a primate (Mandrillus leucophaeus) and the northern white rhinoceros (Ceratotherium simum cottoni; Ben-Nun et al. 2011; Korody et al. 2017).
- Freezing tissue biopsy samples for later initiation of cell culture (i.e., “tissue piecing,” Fig. 3) is described in Houck et al. (1995), Gamble (2014), and Houck et al. (2017, protocol 24.11); herein we summarize this method.
- Tissue is collected in vials containing cell culture media with antibiotics and held at 4 °C (or room temperature if refrigeration is not available); ideally tissues are stored in media for less than three days but can potentially be stored up to 10 days if no contamination occurs.
- Under aseptic conditions tissue is then minced into 1 mm³ fragments and placed in cell culture medium containing 10% DMSO and either transferred to a primed LN2 dry shipper for short-term storage during transport or placed directly into a long-term LN2 storage.
- Tissue prepared this way and kept in LN2 can be stored indefinitely and later transported to a lab with experience in tissue culture to establish cell lines.
- ↑ Zimkus, B.M. and L.S. Ford. 2014. Genetic resource collections associated with natural history museums: A survey and analysis to establish a benchmark of standards. Pp. 9–44 in DNA Banking for the 21st Century. Proceedings of the U.S. Workshop on DNA Banking (W.L. Applequist and L. Campbell, eds.). William L. Brown Center, St. Louis, Missouri. 187 pp.
