Difference between revisions of "Tissue Sample Collection"
BredaZimkus (Talk | contribs) (→Tissue Preservation Methods) |
BredaZimkus (Talk | contribs) (→Tissue Preservation Methods) |
||
| Line 123: | Line 123: | ||
# Tissue prepared this way and kept in LN<sub>2</sub> can be stored indefinitely and later transported to a lab with experience in tissue culture to establish cell lines. | # Tissue prepared this way and kept in LN<sub>2</sub> can be stored indefinitely and later transported to a lab with experience in tissue culture to establish cell lines. | ||
[[File:Slide29.jpeg|center|Comparison of most commonly used preservation methods by Zimkus (unpublished).]] | [[File:Slide29.jpeg|center|Comparison of most commonly used preservation methods by Zimkus (unpublished).]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 18:48, 13 May 2019
Contents
Statement of Purpose
These links and documents contain information about best practices for tissue sample collection.
Introduction
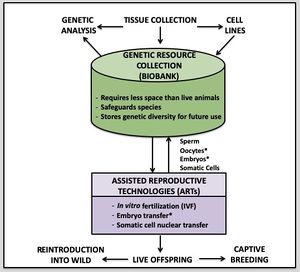
Contributors
Major editor: Breda Zimkus. Original content generated during The American Society of Ichthyologists and Herpetologists (ASIH) Annual Joint Meeting - 2016, during an iDigBio sponsored workshop by the following individuals participating in the "Field to Database" Group of the aforementioned workshop: Breda Zimkus, Cesar Aguilar, Ben Frable, Meredith Mahoney, Zachary Randall, and David Wernecke.
Vial Considerations
One area of immense variability is with the number of vial types used in natural history collections [1].
- Samples stored using LN2 must have vials that are rated for use with cryogenic temperatures, preferably made of polypropylene with screw-top caps. Glass vials are problematic when frozen because of their fragility when handling and vulnerability to cracking when stressed, and their use with LN2 is unacceptable because leakage into the vials can lead to the vessels exploding.
- Pop-off lids are not recommended because this vial type can easily open on its own.
- Vials can have threads located internally or externally on the vial opening; both vial types exhibit advantages and disadvantages.
- - Externally-threaded closures promoteb more sterile conditions because internal threading can allow contaminants to enter if the cap is placed on an unclean surface when removed, but these vials can be susceptible to cracks and loss of air-tightness, which can lead to sample dessication or oxidation (Corthals and DeSalle 2005, Corthals 2006).
- -Internally-threaded vials might allow increased storage capacity, depending on the vial selected, but users also suggest that material can be trapped within the threads of this vial type (Johnson 1999).
- Some manufacturers suggest that vial caps that incorporate a silicone gasket or O-ring (internally or externally threaded) are ideal for vapor-phase LN2 freezing because the seal is enhanced; however, there are some concerns:
- - Care must be taken if the vial cap is over-tightened because the gasket can become distended.
- - The presence of gaskets or O-ring might improve the initial performance of seals but can be problematic when used with some alcohols (e.g., ethanol) because some gasket material, such as silicone, is vapor permeable.
- When working with liquid-phase nitrogen cold storage, extra caution must be taken because the accidental entrapment of liquefied nitrogen inside the vial leads to pressure build up and, upon removal, rapid vaporization of the liquid can result in leakage or even explosion.
- - As a precaution, vial manufacturers recommend that samples not be immersed in LN2 unless they have secondary containment. Heat-sealing vials into flexible polyethylene tubing is recommended for safe storage in the liquid-phase environment.
Labeling and organizing vials
- Pre-label vials
- -Many researchers include specimen tags inside vials, but paper inside vials not ideal because of possible contamination
- Use alcohol-proof pen
- Use scribe to etch identifying information as back-up
- Organizing vials numerically in boxes is preferable to storage in bags in case writing comes off vials
- Tissue samples should be taken as soon after euthanization as possible
Tissue Collection Methods
Some general recommendations include:
- Ensure that gloves are worn
- Make sure that all instruments and surfaces are sterilized (or use disposable products) between sub-samples
- Collect sample as soon after euthanasia as possible
- -Pre-labeling vials may increase preparation speed
- Fill two sets of tissue vials in the field: one for current project and one for institutional collection (rather than subsampling one vial later).
- Label vial to indicate unique number and tissue type
- - Only place one tissue type in each vial
- - Epigenetic studies require same tissue type for all samples in study (i.e., all muscle)
- Cut/slice up tissue sample, small size allows permeation by preservative (Note: read specific recommendations for preservative provided by manufacturer.)
- Fresh preservative may be needed if in field for extended period of time
- Don’t over-fill vial with tissue, or tissue and preservative (expansion, can interfere with vial threads, etc.)
Tissue Preservation Methods
The methods currently used to preserve genetic material during initial sampling vary widely owing to the sampling location, tissue type, and intended research use (Dessauer and Hafner 1984, Prendini et al. 2002, Bus´ and Allen 2014). A comprehensive treatise of methods to preserve genetic samples is discussed in Nagy (2010). In a survey of genetic resource collections associated with natural history
museums, samples were found to be initially preserved in a variety of different ways [1]- ↑ 1.0 1.1 Zimkus, B.M. and L.S. Ford. 2014. Genetic resource collections associated with natural history museums: A survey and analysis to establish a benchmark of standards. Pp. 9–44 in DNA Banking for the 21st Century. Proceedings of the U.S. Workshop on DNA Banking (W.L. Applequist and L. Campbell, eds.). William L. Brown Center, St. Louis, Missouri. 187 pp.