Access and Benefit-Sharing (Nagoya Protocol and the CBD)
Contents
- 1 Statement of Purpose
- 2 Contributors
- 3 How are the Convention on Biological Diversity and the Nagoya Protocol Connected?
- 4 Scope
- 5 The Nagoya Protocol In Brief
- 6 ABS Clearing-House
- 7 National Focal Points and Competent National Authorities
- 8 Access Obligations
- 9 Benefit-sharing Obligations
- 10 Compliance Obligations
- 11 Monitoring User Compliance
- 12 Guidance for Compliance: Codes of Conduct, Guidelines and Best Practice and/or Standards
- 13 Harmonizing Documents: Model Contractual Clauses
- 14 Glossary of Terms and Abbreviations
- 15 Convention on Biological Diversity and Nagoya Protocol texts
- 16 Links
Statement of Purpose
These links and documents contain information about the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization (NP), which is a supplementary agreement to the Convention on Biological Diversity (CBD). Information on Parties to the Nagoya Protocol, including date of signature and status of ratification, acceptance, approval or accession, can be found on the Access and Benefit-sharing Clearing-House (ABSCH).
Contributors
Kate Davis, Dirk Neumann, Breda Zimkus
How are the Convention on Biological Diversity and the Nagoya Protocol Connected?
The CBD, which came into force 29 December 1993, reaffirmed that States hold sovereign rights over their biological resources, in accordance with the Charter of the United Nations and the principles of international law. Countries have the sovereign right to regulate or grant free access to Genetic Resources (GR) occurring inside their national territory (i.e., they determine who can collect biological material that contains genetic resources and what users are allowed to do with it). The CBD has three objectives:
- Conservation of biological diversity
- Sustainable use of its components
- Fair and equitable sharing of benefits arising out of the utilization of genetic resources
The NP was developed to implement legal measures in user countries, mainly to ensure compliance with the third CBD objective, fair and equitable benefit-sharing. It establishes a legal framework for access to genetic resources and benefit-sharing (ABS): it requires countries to clarify access procedures, to share benefits that arise from utilization of GR, and to ensure that users comply with provider country ABS laws. The NP came into force on 12 October 2014.
Natural history collections are affected by the CBD, the NP and all of the various national ABS laws that have been developed in response to them. Associations such as SPNHC have an important role to play in helping institutions, curators and researchers understand the issues and their obligations, and can share guidance, codes of conduct, model agreements and experience.
Scope
The NP is applicable to (1) genetic resources that are (2) within the scope of Article 15 of the CBD and to the benefits arising from the (3) utilization of such resources. It is also applicable to (4) traditional knowledge associated with genetic resources within the CBD’s scope and to benefits from their utilization. To clarify points 1-4:
1. A genetic resource is any biological material of plant, animal, microbial or other origin ‘containing functional units of heredity’, of actual or potential value that can be obtained from the wild, domesticated or cultivated; it may be sourced in-situ (where it naturally occurs) or ex-situ (from human-made collections).
2. Article 15 of the CBD contains certain provisions for how genetic resources should be accessed.

- A country holds sovereign rights over the biological resources inside its national boundaries; each country decides how it will regulate access to genetic resources, or if it will instead grant free access.
- Access to genetic resources requires Prior Informed Consent (PIC) of the country providing the resources (the country of origin or a state that has acquired the resources according to this article and acts as country of origin), unless the country determines that it does not.
- Mutually Agreed Terms (MAT) are to be established between provider and user, including terms for sharing benefits arising from the utilization if national access laws call for MAT to be established
- Unfortunately, ‘access’ itself is not defined in the CBD or the NP. In some countries, access essentially means acquiring Genetic Resources, while in others, access is interpreted as using the contained genetic/biochemical properties of Genetic Resources. While the date of access is decisive for ABS obligations in the first case, the date when the material is actually utilized is decisive in the second. Users need to look out for any differing definitions and interpretations that are used in provider country laws and MAT.
3. Utilization means conducting research and development on the genetic and/or biochemical composition of genetic resources, including through the application of biotechnology; thus this definition basically includes all forms of sequencing and biotechnological activities.
4. Traditional knowledge associated with genetic resources (ATK) is not defined in the CBD or the NP.
The NP does not apply to:
- Human genetic resources;
- Areas beyond the limits of national jurisdiction (e.g. open seas, Antarctica);
- Genetic resources used as commodities;
- Genetic resources accessed before the entry into force of the CBD (29 December 1993).
In addition, the NP may not apply in cases where the genetic resources and the purpose of their utilization are covered by certain specialized ABS instruments that are harmonized with the CBD and the NP, if the countries concerned are Parties to these instruments and the planned utilization is outside the scope of the NP. For example, genetic resources that are used for research on food or agriculture and are part of the multilateral system between Parties to the International Treaty on Plant Genetic Resources for Food and Agriculture (ITPGRFA) are not covered by the NP. This means that peas could be used for specific research questions that are excluded under ITPGRFA (mainly food security), but may not be used to explore the antibacterial potential of pea seeds and how these properties could be used for drug development.
In general the NP applies to genetic resources and ATK acquired from a Party to the Protocol since 12 Oct 2014, or later, depending when the respective country became a Party and established applicable ABS measures. However, some countries established access legislation before the NP, in response to the CBD and refer to the dates upon which those measures came into force. As such, and from the perspective of contractual laws, national ABS laws that pre-date the NP are legally-binding (even though their compliance may not be enforced outside that country). For collections holding material in ex-situ conditions from providers with such pre-NP laws in place, this could be also be understood as good opportunity that help strengthen trust with the respective providing countries and for emerging or existing (research) collaborations in this country.
It is advisable that Providers and User seek clarity and consent (as far is possible) during negotiations and before utilization starts.
The Nagoya Protocol In Brief
- Legally binding supplementary agreement to the CBD for countries that ratify this Protocol
- Adopted on 29 October 2010, in Nagoya, Japan and entered into force on 12 October 2014, 90 days after the 50th ratification
- In principle applicable to the genetic resources within all biological material collected since 12 October 2014 if relevant laws are in place and need to be checked for samples accessed from 12 October 2014 onwards
- Includes 27 clauses in the preamble, 36 articles containing operative provisions, and one annex with a list of possible monetary and non-monetary benefits
- Definitions are rather broad and unspecific to be as inclusive as possible (see Glossary of Terms and Abbreviations for complete list of terms):
- - Utilization (of genetic resources) means to conduct research and development on the genetic and/or biochemical composition of genetic resources, including through the application of biotechnology
- - Biotechnology means any technological application that uses biological systems, living organisms, or derivatives thereof, to make or modify products or processes for specific use
- - Derivative means a naturally occurring biochemical compound resulting from the genetic expression or metabolism of biological or genetic resources, even if it does not contain functional units of heredity
- Covers the utilization of GR for non-commercial purposes (e.g., taxonomy, conservation) and for commercial development (e.g., pharmaceutical development, industrial biotechnology, agricultural biotechnology, commercial horticulture); essentially, it covers research on the structure, characteristics or purpose of naturally-occurring molecules, as well as their further development.
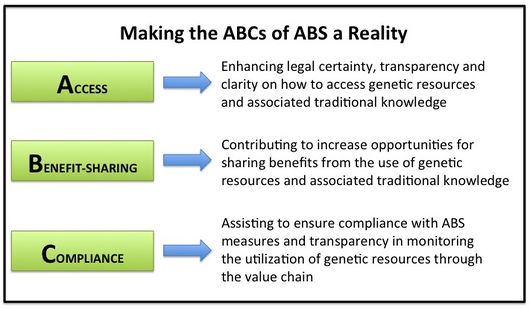
- Sets out core obligations for its contracting parties concerning:
- Access: requires those Parties that require PIC for access to genetic resources and/or traditional knowledge to establish transparent, clear domestic access legislation or regulatory requirements, providing legal certainty, and fair and non-arbitrary rules and procedures on access, clarifying how prior informed consent (PIC) is obtained;
- Benefit-sharing: requires the fair and equitable sharing of benefits arising from the utilization of genetic resources and/or traditional knowledge, upon mutually agreed terms (MAT) between provider and user. Depending on the Providing Country and domestic legislation, additional agreements with indigenous and local communities that hold rights on genetic resources need to be considered or may be required;
- Compliance: obliges Parties to ensure that users under their jurisdiction respect domestic ABS legislation and requirements of providing countries and establishes an international framework for monitoring the utilization of genetic resources.
ABS Clearing-House
The Access and Benefit-sharing Clearing-House (ABSCH) is a web-based platform for information exchange associated with the implementation of the Nagoya Protocol. This website should be the first and most relevant point of information when institutions or researchers plan to access GR or want to look up any country specific information.
Data provided in the country profiles is exclusively provided by the CNAs of the respective Parties and ideally include the following information:
- Checkpoints (i.e., national authorities and contacts associated with ABS)
- Competent National Authorities (CNAs; designated bodies responsible for granting access or issuing evidence that access requirements have been met)
- Contact information for National Focal Points (NFPs; designated body responsible for providing information on national legislation, rules, and procedures for access to genetic resources and ATK, and relevant authorities and stakeholders)
- Relevant legislative, administrative, and policy measures (e.g., act, decree, guidelines, regulations, rules associated with ABS)
- National websites or databases (e.g., guidance for foreign users seeking access to genetic resources, national Clearing-House mechanisms)
- Internationally Recognized Certificates of Compliance (IRCC; generated by the ABSCH when provider countries submit an permit or equivalent that they have issued for access)
National Focal Points and Competent National Authorities
It is up to each Party to decide which national institution they wish to designate as national focal point (NFP) on access and benefit-sharing (ABS). The NFP is responsible for making information on ABS available to applicants seeking access to genetic resources on the country’s procedures for obtaining PIC and establishing MAT. For access to associated traditional knowledge, where possible, the NFP provides information on the procedures for gaining the PIC of indigenous and local communities and establishing MAT. The NFP is the primary contact between the country and the Secretariat of the Convention on Biological Diversity.
Each Party must also designate at least one competent national authority (CNA) on ABS. The CNA has the mandate to determine, authorize, and certify access in accordance with national ABS frameworks. Unlike the NFP, which is responsible for sharing information on ABS procedures, the CNA is responsible for advising on the applicable access procedures and requirements.
It is not mandatory to have both an NFP and a CNA (or CNAs). A Party is free to designate only an NFP that will also serve as and carry out the responsibilities of a CNA – or vice versa. Some countries have established several competent national authorities (CNAs) and require consultation of relevant stakeholders. For example, researchers that wish to collect in protected rainforests with indigenous communities may need to contact several different ministries.
When seeking information on intended access, it is advisable (especially for users utilizing inside the European Union who need to demonstrate their own due diligence under the EU Regulation) for researchers to document their complete communication with the respective NFP. If a NFP fails to respond to repeated requests, evidence of the communication supports the position of institutions and researchers regarding their efforts to meet their diligence obligations under the Regulation. In the interim, it is advisable to pause research plans.
Access Obligations
Countries have sovereign rights to determine whether and how they will regulate access to their genetic resources. The CBD notes that each Party ‘shall endeavour to create conditions to facilitate access to genetic resources for environmentally sound uses by other Contracting Parties and not to impose restrictions that run counter to the objectives of this Convention.’ The NP does not mention facilitated access – but it does require those countries that choose to regulate access, and require prior informed consent (PIC), to take measures to:
- Provide for legal certainty, clarity and transparency in its domestic ABS legislation or regulatory requirements; this does not mean that they must grant access in all cases, but that in principle legal access is possible;
- Establish clear rules and procedures for requiring and establishing PIC & MAT between providers and users for access and benefit-sharing;
- Provide for issuance of a ‘permit or its equivalent’ as evidence of the decision to grant PIC and the establishment of MAT, and notify the ABS Clearing-House (the permit-or-equivalent is used to generate an internationally recognized certificate of compliance – IRCC).
Countries also must:
- Designate National Focal Points to provide ABS information and Competent National Authorities to grant access or issue evidence that access requirements have been met;
- Provide updated information of national contact points, relevant laws and their applicability on the ABSCH website;
- Take measures to ensure that, for traditional knowledge associated with genetic resources, access is with the PIC or approval and involvement of the indigenous and local communities that hold the knowledge, and that MAT have been established;
- Create conditions to promote and encourage research contributing to biodiversity conservation and sustainable use, including through simplified measures on access for non-commercial research purposes (i.e., NP Article 8), taking into account the need to address a change of intent (to commercial purposes);
- Pay due regard to cases of present or imminent emergencies that threaten human, animal or plant health;
- Consider the importance of genetic resources for food and agriculture for food security.
Benefit-sharing Obligations
Domestic-level benefit-sharing measures aim to:
- Ensure that users will share with providers benefits arising from the utilization of genetic resources, as well as subsequent applications and commercialization, in a fair and equitable way.
- Promote the fair and equitable sharing of benefits from the utilization of traditional knowledge associated with genetic resources with indigenous and local communities that hold the knowledge
Benefit-sharing is subject to mutually agreed terms (MAT) (see Glossary of Terms and Abbreviations) between providers and users, and benefits may be monetary or non-monetary.
Monetary and Non-monetary Benefits
The Annex of the Nagoya Protocol contains an indicative, non-exhaustive list of monetary and non-monetary benefits. The many benefits likely to be relevant to natural history collections are highlighted below.
Monetary benefits may include:
- Access fees/fee per sample collected or otherwise acquired
- Commercial products
- Joint ventures
- Joint ownership of relevant intellectual property rights
- Milestone payments
- Licensing fees in the case of commercialization
- Research funding
- Royalties
- Salaries
- Special fees to be paid to trust funds supporting conservation and sustainable use of biodiversity
- Up-front payments
Non-monetary benefits may include:
- Access to information relevant to conservation and/or sustainable use of biological diversity, including biological inventories and taxonomic studies
- Admittance to ex situ facilities of genetic resources and to databases
- Collaborative activities and research
- Contributions to the local economy
- Education
- Human resources for capacity-building and/or enforcement of access regulations
- Institutional capacity-building
- Institutional and professional relationships
- Joint ownership of relevant intellectual property rights
- Knowledge transfer to the provider of the genetic resources
- Material resources for capacity-building and/or enforcement of access regulations
- Participation in product development
- Research directed towards priority needs (e.g., health and food security)
- Research exchange
- Research partnerships
- Social recognition
- Technology transfer to the provider of the genetic resources
- Training related to genetic resources
Compliance Obligations
The Protocol also requires its Parties to develop and enforce measures to support compliance with the domestic legislation or regulatory requirements of the Party providing genetic resources (and contractual obligations reflected in mutually agreed terms) in cases where the genetic resources are utilized in another country. Parties must:
- Provide that genetic resources utilized within their jurisdiction have been accessed in accordance with the applicable access laws and that prior informed consent, and that mutually agreed terms have been established, as required by the other Party. NOTE: To demonstrate due diligence, it is appropriate for collections and researchers to also document – in case of free access – that the absence of agreements such as PIC & MAT is consistent with the access requirements at the date of accessing the samples.
- Take measures to monitor the utilization of genetic resources after they leave a country, including the designation of possible checkpoints at stages of the value-chain: research, development, innovation, pre-commercialization and commercialization
- Cooperate in cases of alleged violation of another Party’s requirements
- Encourage contractual provisions on dispute resolution in mutually agreed terms
- Ensure an opportunity is available to seek recourse under their legal systems when disputes arise from mutually agreed terms
- Take measures regarding access to justice as appropriate
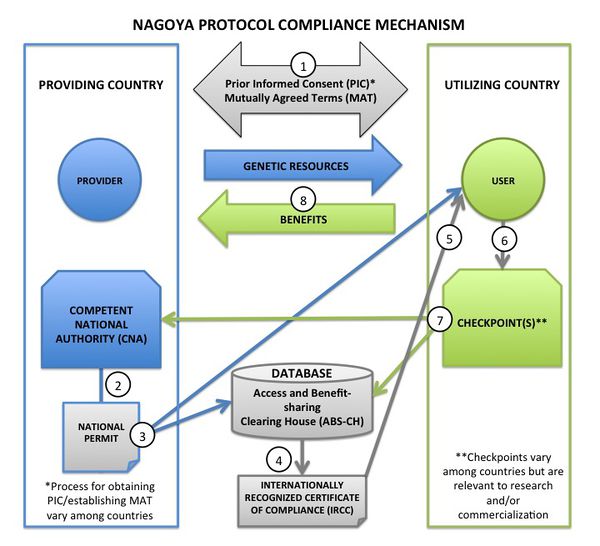
Monitoring User Compliance
The example compliance mechanism schematic (shown below) shows the possible steps included in the compliance mechanism. NOTE: procedures are different in each country and are not harmonized, and not all Nagoya Parties have yet designated checkpoints or issued IRCCs.- In general, the user obtains PIC (if required) and establishes MAT if required by national law (e.g., PIC might be granted by the national or state government or by local communities, MAT might be negotiated with the relevant government contact or with communities or scientific partners), setting out intended use (non-commercial and/or commercial). The ABSCH includes details of how to proceed and the appropriate agents or agencies to contact.
- The providing country's Competent National Authority (CNA) issues a permit or its equivalent to the user, or issues evidence that the user obtained PIC as required (e.g., from local communities); ‘national permit’ in the diagram.
- The CNA publishes a record for the national permit on the ABSCH, and user conducts activity under the national permit. (Note: other non-ABS permits may still be necessary!)
- The ABSCH generates an internationally recognized certificate of compliance (IRCC), which includes a unique identifier for tracking.
- Genetic resources can be utilized, and benefit-sharing occurs as a result of the genetic resources being utilized, as established in the mutually agreed terms. (Note: benefit-sharing may occur before, during and after the time period when genetic resources are being used, depending on MAT.)
- At certain stages (variable in each country, e.g., the use of public funds, application for intellectual property rights, putting a finished product on the market), the user provides information related to PIC, the source of the genetic resources, the establishment of MAT, and/or to the utilization of genetic resources, to a checkpoint agency within the utilizing country. (Note: if there is an IRCC, the IRCC number provides much of this information.)
- The checkpoint communicates information to the national authorities of the providing country and the ABSCH (as appropriate; sometimes information is confidential), via a checkpoint communique, including IRCC number if available.
Guidance for Compliance: Codes of Conduct, Guidelines and Best Practice and/or Standards
Different research disciplines utilize genetic resources in quite different ways, for different purposes and with different analytical and curation systems. It is useful for institutions to develop and share practical procedures for colleagues and internal and external researchers to help them with compliance. The NP creates a place for these voluntary initiatives. It requires Parties to encourage, as appropriate, the development, update and use of such voluntary codes of conduct, guidelines and best practices and/or standards in relation to ABS.
European Union (EU) Regulation No. 511/2014] (with further details set out in Implementing Regulation (EU) 2015/1866) allows sectors to apply for EU recognition of such best practices for utilization that happens inside the EU. If a collection is known to follow a recognized best practice, the competent authority in that country is likely to regard that collection’s activities with more trust, and perhaps conduct fewer checks. The governing body of the NP will periodically take stock of their use and consider the adoption of specific codes, etc.
Several ABS codes of conduct and best practices have been developed by various networks of ex situ collections, including:
- CETAF Code of Conduct and Best Practices developed by the Consortium of European Taxonomic Facilities, a European network of museums, botanic gardens and other taxonomic institutions
- GGBN Code of Conduct and Best Practices developed by the Global Genome Biodiversity Network for ex situ collections holding genome-quality material; harmonized with the CETAF package, reflecting significant membership overlap with CETAF and SPNHC
- The Principles on ABS for botanical institutions (not updated post-Nagoya, but still functional)
- International Plant Exchange Network (IPEN) developed by European botanic gardens
- Transparent Users-friendly System of Transfer (TRUST) for microbial collections
- Microbial Resource Research Infrastructure (MIRRI) best practices for microbial collections
These collections-designed codes and best practices each provide practical guidance on how to acquire, use, curate and supply biological material and genetic resources in compliance with the CBD and the NP. The CETAF and GGBN best practices also provide detailed guidance on data management practices and policy areas that need to be considered, and model documents (see also Model contractual clauses, below). TRUST and IPEN provide whole systems for exchanging material. TRUST covers microbial collections and builds upon Micro-Organisms Sustainable use and Access regulation International Code of Conduct (MOSAICC); IPEN covers living plant collections and non-commercial uses only.
Harmonizing Documents: Model Contractual Clauses
The NP makes room for and encourages the development of contractual clauses for different sectors and research disciplines. The governing body of the Protocol will periodically take stock of their use.
Model terms and (cross) sectoral contractual clauses may be constrained by the provider country’s laws (established by the country, ideally with input from sectors), or sectors may develop the models themselves and offer them for provider consideration in negotiations.
The use and sharing of such model contract terms, and even model agreements, by networks will facilitate research collaborations. In particular, harmonized (or even standard, where possible) Material Transfer Agreements (MTAs) make it simpler for collections and researchers to transfer material.
- Model MTAs: For natural history collections, CETAF and GGBN have developed three model MTAs that can be used for transfers (i.e., when supplying material, when receiving material if the provider or donor does not have its own supply MTA, when providing temporary transfer like that of a loan). For some institutions, tissue samples and DNA extracts are not loaned because they are consumed during analysis and are not returned. Consequently, traditional loan forms issued by natural history museums may not be appropriate for supply of such material. CETAF and GGBN also provide a model Data Use Statement for use when publishing genetic information or uploading DNA sequences.
- Model Statements of Use: CETAF and GGBN Codes of Conduct and Best Practices also supply a model ‘Statement of Use’ that offers common language for researchers and collections to use during negotiations for PIC and MAT, explaining in a certain amount of detail how material will be used once it is back at the institution. The Statement of Use can be customized depending on the actual research activities of each institution (e.g. all botanic gardens have living collections so need to explain how they use plants within, while museums may not have any living collections, or may use them quite differently). However, the more harmonized the language of the Statements of Use are, the more recognized they will be by providers, and the useful they will become to the collections community.
- Model ABS Agreements: the Swiss Academy of Sciences has developed (and is updating) a Sample Agreement for Non-Commercial Research, which can be used by providers and users as a basis for negotiating MAT.
Glossary of Terms and Abbreviations
- ABS Clearing-House (ABSCH): online platform for information-sharing developed under the Convention on Biological Diversity; it includes national contacts, national legislation, internationally-recognized certificates of compliance and other matters relevant to ABS and the implementation of the Nagoya Protocol; https://absch.cbd.int/.
- Access to genetic resources: ‘Access’ is not defined in the CBD or in the Nagoya Protocol and thus is interpreted and defined quite differently in national ABS laws. The European Union Regulation 511/2014 defines access as ‘acquisition of genetic resources or of traditional knowledge associated with genetic resources in a Party to the Nagoya Protocol’ (= obtaining of biological samples). Brazilian law 13.123/2014 defines access as ‘research and technological development carried out on a genetic heritage sample’ (= the action of utilizing samples). The latter definition means that the ‘start date’ of the law’s coverage is when the samples are utilized for the first time, independent of the date of their acquisition. National laws also differ in the coverage of access, e.g. some refer to access to biological resources (as in several African countries), rather than genetic resources, and some even widen the concept beyond physical material to information, e.g. Brazilian law refers to access to genetic heritage, which includes information. The CETAF Best Practices (Annex 3) define access as: ‘the acquisition of Genetic Resources or of Traditional Knowledge associated with Genetic Resources from a Providing Country.’
- Associated Traditional Knowledge (ATK): information associated with genetic resources held by indigenous and local communities, as well as to genetic resources held by indigenous and local communities where the rights of these communities over these resources have been recognized. Note: there is currently no generally accepted definition of traditional knowledge at the international level. WIPO defines it as “knowledge, know-how, skills and practices that are developed, sustained and passed on from generation to generation within a community, often forming part of its cultural or spiritual identity.” It also notes “TK in the narrow sense refers to knowledge as such, in particular the knowledge resulting from intellectual activity in a traditional context, and includes know-how, practices, skills, and innovations.” (http://www.wipo.int/tk/en/tk/). The Nagoya Protocol and EU Regulation cover TK associated with Genetic Resources (TKaGR), not TK as a separate element. (Consult CETAF Best Practices Annex 3.)
- Benefits: monetary and/or non-monetary benefits arising from the use of genetic resources, including research and/or development; the NP Annex provides a list of examples
- Biotechnology: any technological application that uses biological systems, living organisms, or derivatives thereof, to make or modify products or processes for specific use (NP definition, referencing Article 2 of the CBD)
- Competent National Authority (CNA): the individual or national body (e.g., coordination council, department, institute, ministry) authorized to sign ABS agreements
- Convention on Biological Diversity (CBD): multilateral treaty known informally as the Biodiversity Convention; it entered into force on 29 December 1993
- Derivative: a naturally occurring biochemical compound resulting from the genetic expression or metabolism of biological or genetic resources, even if it does not contain functional units of heredity (NP definition)
- Ex situ condition: means condition where genetic resources are found outside their normal ecosystem or habitat inside or outside of the original sourcing country, such as in botanical gardens or seed banks, or in commercial or university collections, either inside or outside the original Providing Country
- Genetic material: any material of plant, animal, microbial or other origin containing functional units of heredity (CBD definition)
- Genetic resources: genetic material of actual or potential value (CBD definition)
- Internationally Recognized Certificate of Compliance (IRCC): a record generated when the "Competent National Authority" publishes a permit or its equivalent (outining PIC and MAT) on the ABS Clearing-House that provides legal surety of the genetic resources covered
- In situ sources: resources obtained from the provider country (e.g., fieldwork)
- Material: refers to the items included within the Material Transfer Agreement
- Material Transfer Agreement (MTA): an agreement between two institutions that outlines the terms and conditions for transferring specimens or samples, including genetic material.
- Mutually Agreed Terms (MAT): an agreement between the providers of genetic resources and users on the conditions of access and use and the benefits to be shared between both parties. Note: MAT applies to third-party uses (e.g., loans, gifts) and may apply to specimens in perpetuity; new uses may need to be renegotiated.
- National Focal Point (NFP): responsible for making information on ABS available, informs potential users of the procedures that are to be followed in applications for access to genetic resources and traditional knowledge associated with genetic resources, and is also responsible for sharing information on competent national authorities (CNAs) and relevant stakeholders
- Prior Informed Consent (PIC): permission given by the competent national authority of a provider country to a user prior to accessing genetic resources, in line with an appropriate national legal and institutional framework (i.e., what a user can and cannot do with the material). Note: new uses may need to be renegotiated.
- Recipient: the organization to whom the Supplier sends the Material.
- Supplier: the party providing the Material
- Utilization (of genetic resources): to conduct research and development on the genetic and/or biochemical composition of genetic resources, including through the application of biotechnology (NP definition)
Convention on Biological Diversity and Nagoya Protocol texts
- Secretariat of the Convention on Biological Diversity. 1992. Convention on Biological Diversity. Secretariat of the Convention on Biological Diversity, Montreal, Canada. https://www.cbd.int/convention/text/.
- Secretariat of the Convention on Biological Diversity. 2011. Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the Convention on Biological Diversity. Secretariat of the Convention on Biological Diversity, Montreal, Canada. https://www.cbd.int/abs/text/default.shtml.
Links
ABS Information and Explanatory Guidelines

- The Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization
- Access and benefit-sharing 2-page factsheet
- Nagoya Protocol 2-page factsheet
- An Explanatory Guide to the Nagoya Protocol on Access and Benefit-sharing
- The Principles on Access to Genetic Resources and Benefit-Sharing
- ABS Learning Tool (Botanic Gardens Conservation International)
- ABS Information Forum
- Talking Points for the Nagoya Protocol
- The Nagoya Protocol Learning Portal
Community Standards
- Global Genome Biodiversity Network (GGBN) Guidance: Best Practice for Access and Benefit-Sharing (2014; biorepositories for genome quality material)
- Consortium of European Taxonomic Facilities (CETAF) Code of conduct and best practices (2018; taxonomy-focused natural history museums and botanic gardens)
- Principles on Access to Genetic Resources and Benefit-Sharing and Common Policy Guidelines (2001; botanic gardens)
- Plant Exchange Network (IPEN) (2001; botanic garden living collections)
- Utilization of genetic resources in academic research: a good practice guide (developed by Swiss Academy of Sciences)
- Transparent Users-friendly System of Transfer (TRUST; microbial collections; updated 2016)
- Microbial Resource Research Infrastructure (MIRRI) Best Practices (2016; microbial collections)
- The ABS Management Tool (ABS-MT; revised 2012; provider governments and communities and cross-sectoral users)
Example Agreements and Policies Related to ABS
- Sample ABS Agreement for Non-Commercial Research (2010; Swiss Academy of Sciences, ed.)
- National Museum of Natural History (NMNH), Smithsonian Institution: Access and Benefit Sharing Policy on Genetic Resources
- Global Genome Biodiversity Network (GGBN) Standard Material Transfer Agreements (Standard MTA for provision of material with no change in ownership; Standard MTA for provision of material with change in ownership; Standard MTA for Receipt of material with change in ownership)
- CETAF Standard MATERIAL TRANSFER AGREEMENT (MTA 1) for PROVISION OF MATERIAL with no change in ownership
- CETAF Standard MATERIAL TRANSFER AGREEMENT (MTA 2) for PROVISION OF MATERIAL with change in ownership
- CETAF Standard MATERIAL TRANSFER AGREEMENT (MTA 3) for RECEIPT OF MATERIAL with change in ownership
- ABS Implementation examples compiled by Kate Davis, Consultant ABS Advisor to BGCI.
- Guidance document on the scope of application and core obligations of Regulation (EU) No 511/2014 of the European Parliament and of the Council on the compliance measures for users from the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilisation in the Union
Conference Presentations
- Neumann, D. Transfer of specimens under the Nagoya Protocol & ABS. SPNHC 2017 Annual Meeting Shipping Workshop, Denver.
- Zimkus, B. The ABCs of ABS: The Nagoya Protocol and Its Relevance to U.S. Natural History Collections, Collections Management and Research. Oral Presentation at Ecological Society of America Workshop (October 2017).
- Zimkus, B. New Horizons for Research and Collections: The Nagoya Protocol on Access and Benefit-Sharing. Oral Presentation at BCoN Workshop Hosted by Museum of Comparative Zoology (March 2018).
Further Reading
- Neumann, D., C.H.C. Lyal, J. Bodgard, C. Lohne, A. Casina, A. Nivart, C. Williams and P. Giere. 2014. SPNHC Connection 28(2), pp. 40-42.[2]
- Davis, K. and D. Neumann. 2016. ABS for Barcoders, Part 1: Alphabet Soup. Barcode Bulletin 7(1), pp. 6-7. [3]
- Neumann, D. and K. Davis. 2016. ABS for Barcoders, Part 2: Recipes for Compliance. Barcode Bulletin 7(2), pp. 10-11. [4]
- Neumann, D., A.V. Borisenko, J.A. Coddington, C.L. Häuser, C.R. Butler, A. Casino, J.C. Vogel, G. Haszprunar, and P. Giere. 2018. Global biodiversity research tied up by juridical interpretations of access and benefit sharing. Organisms Diversity & Evolution 18: 1. [5]
- Davis, K. and Borisenko, A. 2017. Introduction to Access and Benefit-sharing and the Nagoya Protocol: What DNA Barcoding Researchers need to know. Pensoft Publishers, Sofia, 37 pp. [6]
SPNHC Positions on Digital Sequence Information
- SPNHC views on potential implications of the use of digital sequence information on genetic resources for the three objectives of the Convention. [7]
- Promoting sustainable use and conservation of biodiversity through open exchange of Digital Sequence Information. Joint statement by public and private sector organisations, academic and scientific institutions, data repositories and collections representing a broad range of stakeholders (signed by SPNHC).[8].