Adhesives
Contents
- 1 Contributors
- 2 Introduction
- 3 The use of adhesives in botanical collections
- 4 Adhesives used for mounting, repairs and conservation treatment
- 5 Methylcellulose (MC)
- 6 Hydroxylpropylcellulose (HPC)
- 7 Wheat starch paste (WSP)
- 8 Animal glues: Gelatins, Isinglass
- 9 Poly(vinyl acetate) (PVA)
- 10 Acrylics
- 11 A note on the Archer's plastic adhesive
- 12 Conclusions on the use of adhesives in botanical collections
- 13 Bibliography
Contributors
Introduction
This website deals with adhesives, which are used for mounting, repairs and conservation of herbaria. Although the information is based on research related to botanical material (link), many of these materials may be present in a number of other types of biocultural collections (zoological, ethnographic and other).
The use of adhesives in botanical collections
Adhesives have been used in herbaria from the beginning of their origins to attach plant specimens to the paper support. Many historical herbaria include “ glued” or “ gummed” specimens. In Isagoge in rem herbariam libri duo (1606), the oldest written source on the making of herbaria, Adriaan van den Spiegel provides a detailed recipe for the glue used for mounting: a mixture of bovine glue, ‘hepatic’ aloe extract, and clove powder[1]. Joseph Pitton de Tournefort (1694) recommended the hide glue with the addition of mercury (I) chloride or mercury (II) chloride He also criticized the preparation of this adhesive from a decoction of “Semen contra”, “Absinthe commune” and aloe or “similar drugs”, as these change the color of the plant[2]. From the text, it is also not entirely clear if Tournefort used tested decoctions separately or in a mixture. One of the most influential botanists of all times, Carl Linnaeus used to mount his herbarium specimens with ichthyocolla, which is either translated as isinglass or as fish glue. Both adhesives are made from fish parts, the former from the swim bladders, the latter from skins and bones of non-oily types of fish, but have completely different properties, e.g., fish glue has a lower viscosity, lower pH, and poorer aging resistance than isinglass (Schellmann, 2007; Down, 2015). After over four centuries, direct point or overall adhesion of plants onto the paper support is still one of the most common methods of mounting herbarium specimens, along with strapping and sewing. The same or different adhesives are also used to anchor the straps, attach the labels and paper capsules in the form of envelopes, folders, or packets for fragments of specimens (Bridson & Forman, 1999: 38). Although mounting technique has not changed, conservators and herbarium mounters today can choose from many more types of adhesives, both natural and synthetic. They also have opportunities to test, evaluate, and compare these materials prior to use. The use of particular adhesives in botanical collections has been examined and evaluated in numerous publications (e.g., Croat, 1978; Tillet, 1979, 1989; Clarck, 1986; Egenberg & Moe, 1991; Down, 1999; Collins, 2014). Conservators emphasize the difference between their approach to mounting and that of botanists, and the growing role of the evaluation by conservators of mounting materials. Today it is the conservator’s and/or curator’s duty to introduce in their institution an appropriate adhesive, conforming to conservation quality standards. The choice of adhesive depends on its features, among which the most desirable are compatibility with the object, chemical stability (good aging properties in regard to pH, color, flexibility, strength, dimensions), removability, minimal invasiveness and easiness of application (Down, 2015). It has become clear that the quality of an adhesive may affect the condition of the specimen, and using inappropriate material results in a progressive deterioration of desiccated plants (Clarck, 1986; Egenberg & Moe, 1991). Typical damage derives from adhesive-related uneven tensions, breaking, cracking and crumbling of a fragile specimen, or detachment resulting in a loss. Since there is no perfect adhesive, the choice of mounting material is always a compromise. Another issue, raised several times by Down (1999, 2015), is that from time to time, adhesives, i.e., poly(vinyl acetate) emulsions (PVA), have their formulas changed or their production stops. Thus, once a particular type of adhesive is approved for use by the conservator or curator, it should be regularly re-evaluated.
Adhesives used for mounting, repairs and conservation treatment
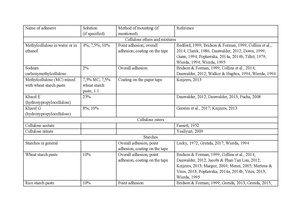
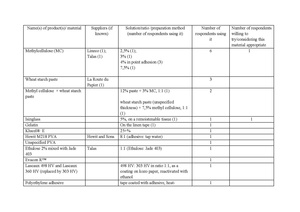
In 2017, a survey was conducted among conservators and curators of the botanical collections[3]. Following data is a result of this research.
Table 1Some respondents emphasized the necessity to avoid the direct contact of the adhesive and the plant material, recommending the use of tapes. However, as most ready-made tapes are coated with adhesive on the whole surface, the issue of the direct contact of the adhesive and a plant specimen also occurs in this manner of mounting. Although the bonding agent is to be activated only on the edges of the strap, there is always a chance that a temporarily elevated humidity (during mounting, handling, or storage) will also work as the activator of the remaining part of the tape. Therefore, mounting tapes were incorporated into the results as another source of adhesive that might come into direct contact with the specimen. Another factor to consider in this case is the emission of volatile organic compounds (VOC), typical for PVA adhesives (discussed below) that may contribute to the degradation of cellulose.
Methylcellulose (MC)
The use of methylcellulose (MC), a cellulose ether, is well established in the conservation world (Down, 2015). It is available in various types, grades and molecular weights, which influence their properties such as viscosity and flexibility. It is worth noting that most respondents did not specify the type of MC they are using. In many European countries, conservators frequently use Tylose MH. This product is commonly named methylcellulose or “ cellulose glue”, but chemically it belongs to another group of cellulose ethers, methylhydroxyethylcellulose (MHEC or HEMC). MHEC is obtained by introducing a methoxy group into the cellulose chain and generally has similar properties to MC. It is, however, more hydrophilic, and its gel temperature is higher than that of MC (Yoo & Um, 2013). Methylcellulose is known for forming stable and resilient films and being resistant to biodeterioration (Down, 2015). The bonds are weaker than these of starch paste and gelatin, but they retain strength better upon aging (Down, 2015). According to the respondents’ comments, MC is a mild (“ weak” ) adhesive that is highly hydrophilic (holding water) and dries slowly (Nasatto & al., 2015). For this reason, it used to be mixed with other adhesives, such as wheat starch paste or PVAs, to produce stronger and faster drying bonds (Feller & Wilt, 1990; Down, 2015). It also presents no discoloration and no viscosity loss upon aging (Feller & Wilt, 1990). No research was found confirming that films of MC become acidic over time, as one respondent had suggested[4]. As long as it is prepared from powder, MC does not alter the pH of the substrate. Nevertheless, Feller &Wilt (1990) point out that aqueous solutions obtained from the manufacturer might present a wide range of pH (3– 8), depending on the purity of the solution. In the experiment of Baker (1984), MC applied on paper did not affect the pH of the substrate; however, it is susceptible to either acidic or alkaline hydrolysis. Therefore, the acidity of the substrates may contribute to the degradation of methylcellulose (Feller & Wilt, 1990).
Hydroxylpropylcellulose (HPC)
Another cellulose ether, hydroxylpropylcellulose (HPC) Klucel® E, was assessed positively for its flexibility and good aging behavior. It belongs to a group of low-molecular weight (80.000) HPCs, evaluated as having better properties than the high-molecular (up to 11.500.000) Klucels: it is more stable, shows less discoloration, and less molecular weight loss upon aging (Feller & Wilt, 1990). Unlike MC, HPCs are prone to biodeterioration. Generally, Klucels are reversible and retain solubility upon aging (Down, 2015). However, in some studies, a distinctive discoloration caused by Klucel® E was reported (Geiger & Michel, 2005; Michel, 2011). Klucel® E has not been studied regarding its aging properties in relation to conservation purposes.
Wheat starch paste (WSP)
Wheat starch paste (WSP) is another adhesive that is very popular in paper conservation and has a very long history of applications. Pure WSP is a fairly stable adhesive but in pure form is prone to microbial attack and becomes brittle upon aging (Down, 2015). It forms strong bonds, yet, as mentioned before, WSP films are less flexible than the ones of MC. The “reversibility” attributed to WSP can actually be related to its removability (which is not an equal term to “reversibility” in the conservation field, see, e.g., discussions in Horie, 2010 and Down, 2015). Dry WSP films, although not soluble in water, can be removed after swelling in water or with the aid of enzymes (Down, 2015).
Animal glues: Gelatins, Isinglass
Comments concerning animal glues, gelatin and isinglass, point out their flexibility and excellent aging properties. This is confirmed by the good condition of historical specimens.[5] A wide variety of gelatins are available, and their chemical properties depend on the source of collagen and type of processing, which determine the decisive feature factors: molecular weight (MW) and its distribution (Schellmann, 2007). Isinglass was traditionally prepared from sturgeon swim bladders, and is a form of pure gelatin extracted from the inner lining of the air or swim bladders of certain fish species, especially sturgeons (Acipenseridae) (not to be confused with fish glue, made of skins and bones of non-oily types of fish, as mentioned before). Today, it may be extracted from the swim bladders of other species because of the decline in the sturgeon population (Schellmann, 2007; Down, 2015). The MW of mammalian gelatins varies from 110.000 to 168.000, while the MW of isinglass starts at 150.000 and can be as high as 300.000, making it a highly viscous (and penetrating) agent. Its gel temperature is distinctly lower than for other animal glues, allowing a more extended penetration. The setting time and tack development are longer than those of gelatin because of these unique properties (Schellmann, 2007). Isinglass and mammalian gelatins are generally flexible, with the former being more flexible than the latter, and this was acknowledged by the respondents. Isinglass is also more stable upon aging (Schellmann, 2007; Down, 2015). Gelatin and isinglass yield solutions with a pH ranging from 5 to 6.5 and from 6 to 7.5, respectively (Schellmann, 2007). Also, animal glues are quite susceptible to microbial attack, which makes their use inadvisable in tropical climates, and creates a risk of infestation when an accidental increase of relative humidity occurs (Schellmann, 2007; Down, 2015).
Poly(vinyl acetate) (PVA)
It is worth mentioning that the same abbreviation PVA has been used referring to both poly(vinyl alcohol) and poly(vinyl acetate), and today both groups of adhesives are labeled using it (Down, 2015). Among the poly(vinyl acetates), there are subgroups of copolymers: EVA, which is an ethylene and vinyl acetate (VAC) copolymer (with more ethylene than VAC); VAE, which is a VAC and ethylene copolymer (with more VAC than ethylene); VADBM, which is a VAC and di-butyl maleate copolymer; and others. Similarly, within this group, for copolymers of VAC and ethylene (regardless of the ratio), the most popular abbreviation used is EVA (Scheper, 2005). The extensive adhesives study of the Canadian Conservation Institute (CCI), including natural aging, provided data on the chemical stability and aging of several poly(vinyl acetate) and acrylic adhesives (Down, 1996, 2015, 2016; Down & al., 1996). Experiments concerning volatile organic compounds emitted by poly(vinyl acetates) showed that dry PVA films tend to release volatile acetic acid, with the highest rates of emission during the first three months after application, decreasing almost to zero after two years (Down & al., 1996, and updated results in Down, 2014, 2015). Most of the acetic acid is released during setting and drying, and this effect is enhanced by the presence of acetic acid added by the manufacturers to stabilize the dispersion (Down, 2014). The only other acid identified was the formic acid emitted by Elmers® Glue-All PVA (Down & al., 1996). Tests conducted in the British Library (Stevens & al., 2011) confirmed that Evacon- R™ (not tested by CCI) produces volatile organic acids under Oddy test conditions and upon aging (artificial aging tests). The same result was obtained for Hewit M218 PVA during internal British Museum testing (British Museum, 2014– 2018). Acetic and formic acids contribute to the decrease of pH and acceleration of the acid hydrolysis of cellulose, especially if an artifact (or a herbarium sheet) is housed in an enclosure (Dupont & Tétreault, 2000). In CCI tests, the acetic acid emission was generally related to the acidity of the adhesive itself (the lower the pH, the higher the emission); i.e., tests confirm what one respondent said, that Weldbond® PVA is an example of a low-pH, high-acetic-acid-emitting adhesive (Down & al., 1996; Down, 2014). Many PVA adhesives are indeed acidic, both as a dispersion and as a dry film; e.g., Bulldog Grip 20 Minute, Elmer’ s® Glue-All, Gaylord MagicMend and Weldbond® (Down, 2014), to mention only the ones that were found in the literature study (Table 1). Dispersions tend to become more acidic upon storage; that is why it is not advisable to store large quantities of PVA for longer than one year (Down & al., 1996; Down, 2014, 2015). However, increasing acidity of dry films upon aging (as suggested in the survey) does not apply to most of the adhesives tested by the CCI. For many products, the pH remained at the same level even after 27 years of aging, and in some cases, the pH was slightly rising with time. An exceptional case is Jade 403, which is acidic as a dispersion (pH = 5.8 before and 5.62 after aging), but neutral as a dry film, also after aging (Down, 2014). The reversibility of PVA was not evaluated in the CCI tests, but previous studies of Baer, Phelan and Indictor (Phelan & al., 1971; Baer & al., 1978) investigated solubility and swelling as factors enabling the adhesive removal. Results showed that none of the products tested is entirely reversible, and for this reason they are not recommended for the treatment of paper as a porous material (Baer & al., 1978). There are no published recent studies of removability of PVA adhesives, examining products that are currently available. However, despite the manufacturers’ declarations of reversibility, this group of adhesives is regarded in conservation as not removable from cellulose-based substrates, and therefore, a contaminating factor.
Acrylics
The only acrylic adhesive that was reported in the survey is a mixture of Lascaux buthyl acrylate/methyl methacrylate copolymers, 498 HV and 360 HV. 498 HV is known for forming strong bonds, and 360 HV for flexibility and retaining tackiness as a dry film. In 2012, the manufacturer replaced 360 HV with another grade, 303 HV, which is a 2-ethylhexyl acrylate/ethyl acrylate copolymer and is permanently tacky (Down, 2015). The mixture of two grades provides a strong and very elastic bond (Kite&Thomson, 2006). Several acrylics were included in the CCI adhesives testing, i.e., Lascaux 360 HV, and Rhoplex™ 235, which has a similar formulation to Lascaux 498 HV (Down & al., 1996). Acrylic adhesives are generally lightfast. However, light aging causes some yellowing. In the CCI tests, 360 HV was the only acrylic that emitted a low amount of acetic acid (Down, 2014). In internal British Museum materials testing, 303 HV passed Oddy tests (British Museum, 2014– 2018). Removability of some acrylic adhesives was studied by the CCI in the project concerning adhesive tape and heat-set tissues (Down & al., 2011). Tapes coated with Lascaux 498 HV and 360 HV proved to be removable either mechanically, with water or organic solvents (i.e., ethanol or acetone). The latter, however, required soaking the sample in the solvent, which is not always possible as a treatment method. The removability was assessed in regard to the tape removal, but the purity of the paper fibers after treatment was not investigated. Tests also showed that removability depends on the type of paper and may decrease upon aging. Therefore, the reversibility of this group of adhesives is also questionable, and assessing this would need further studies.
A note on the Archer's plastic adhesive
The suggestion to test the Archer’s adhesive[6] could not be addressed in this research since the ingredients of this adhesive are no longer in production. Based on the formulas listed by Croat (1978), the author tried to find the following resins: Dow resin 276-V2 [poly(alpha-methyl)styrene resin, manufactured by Dow Chemical Company, Michigan, U.S.A.], Amoco Resin 18-210 (by Amoco Chemicals Corp, Indiana, U.S.A.) and Hercules Piccolastic™ A5/A25 (hydrocarbon styrene monomer resin, manufactured by Hercules, Delaware, U.S.A.). The resins seem either not available on the market (as in the case of Dow and Amoco resins) or unavailable for an individual buyer (Piccolastic™, now produced by Eastman Chemical Company, Tennessee, U.S.A.). Therefore, it would be difficult to recreate the formula of Archer’s adhesive in any version. Moreover, differences in formulas indicate that each time, the adhesive may present different properties. The other reason is that the original Archer’s formulation proved to be highly toxic, as it includes volatile organic compounds, such as methanol and toluene (Croat, 1978; Bridson & Forman, 1999: 39; Down, 1999). Among the side effects experienced by the mounters during their work were nausea and dizziness (Croat, 1978); however, the long-term VOC emission and toxicity of Archer’s adhesive has not been tested, and health hazards for those working with collections mounted with this mixture are currently not known.
Conclusions on the use of adhesives in botanical collections
Compared to the outcome of the literature study, responses in the survey[7] indicate a rather limited range of acceptable materials, focusing on cellulose ethers, animal glues, wheat starch paste, and just a few examples of PVA adhesives. To some extent, this is probably due to the selection of respondents who present a specific conservator’s approach to the choice of materials, where problems of reversibility, off-gassing, pH, and the long-term stability of the applied adhesive are major concerns. Today’s knowledge about the aging of adhesives and harmful ingredients in their formulations is much more profound and documented than some decades ago. Nevertheless, alterations of the formulations of once assessed products make it difficult to keep these assessments always up-to-date. Compared to the literature study, the survey results show the shift in the treatment of herbaria as sources of information. The versatile use of herbarium material in different domains of science and the development of DNA examination techniques have contributed to a change of approach to herbarium-making techniques. The choice of adhesive is dictated not only with regard to its working properties but also its long-term chemical stability and compatibility with the plant material. The current discussion on adhesives addresses the problems of accelerating the degradation of cellulose-based materials and the contamination of herbaria by irreversible or rather not removable material. The survey results confirm that, as Jane Down put it two decades ago (1999: 221), “ the perfect adhesive for herbarium collections is still elusive”. Each recommended adhesive received also a negative or ambivalent review. /link/ It also suggests that there is a constant need for adhesive testing and evaluation on many levels of their properties and aging resistance.
Bibliography
Archer, W. 1950. New plastic aid in mounting herbarium specimens. Rhodora 52 (624): 298-299.
Baer, N.S., Indictor, N. & Phelan, W.H. 1978. An evaluation of poly(vinyl acetate) adhesives for use in paper conservation. Restaurator 2 (2): 121-138. https://doi.org/10.1515/rest.1978.2.2.121
Baker, C.A. 1984. Methylcellulose and sodium carboxymethylcellulose: an evaluation for use in paper conservation through accelerated aging. Stud Conserv. 29 (sup1): 55-59. https://doi.org/10.1179/sic.1984.29.Supplement-1.55
Bedford, D.J. 1999. Vascular plants. Pp. 61-80 in: Carter, D. & Walker, A. (eds.), Care and conservation of natural history collections. Oxford: Butterworth Heinemann.
Bridson, D. & Forman, L. 1999. The herbarium handbook. Kew: Royal Botanic Gardens.
British Museum. 2014-2018. Oddy Test Result Database. https://www.britishmuseum.org/research/publications/research_publications_series/2004/selection_of_materials.aspx (accessed November 17, 2019) stored and available also: https://www.conservation-wiki.com/wiki/Oddy_Test_Results:_Exhibition_Adhesives_and_Tapes (accessed 27 May, 2020)
Clarck, S. 1986. Preservation of herbarium specimens: an archive conservator's approach. Taxon 35 (4): 675-682. https://doi.org/10.2307/1221610
Collins, C., Gregson, J., Yesilyurt, J., Doyle, S., Jacobs, D., Rich, T., Purewal., V. & Young, D. 2014. Standards in the care of botanical materials. http://conservation.myspecies.info/node/35 (accessed 27 May, 2020)
Croat, T.B. 1978. Survey of herbarium problems. Taxon 27 (2/3): 203-218. https://doi.org/10.2307/1220243
Dauwalder, L. 2012. Das Herbarium des Felix Platter. Die Erhaltung eines historischen Buch-Herbariums. MA thesis. Bern: Fachochshule Bern.
Dauwalder, L. 2013. Felix Platter's herbarium: the preservation of a historical bound herbarium. Journal of Paper Conservation 14 (3): 26-32.
DeWolf, G. 1968. Notes on making an herbarium. Arnoldia 28 (8/9): 69-111.
Down, J. 1999. Adhesive research at the Canadian Conservation Institute as it relates to herbarium collections. Pp. 205-223 in: Metsger, D.A. & Byers, S.C. (eds.), Managing the modern herbarium- an interdisciplinary approach. Ontario: SPNHC.
Down, J.L. 2014 The evaluation of selected poly(vinyl acetate) and acrylic adhesives: a final research update. Stud Conserv. 60 (1): 33-54. https://doi.org/10.1179/2047058414Y.0000000129
Down, J.L. 2015. Adhesive compendium for conservation. Ottawa: Canadian Conservation Institute.
Down, J.L. 2016. The effect of modifiers on the stability of a vinyl acetate/ethylene copolymer dispersion, Stud Conserv. 61 (1): 26-45. https://doi.org/10.1179/2047058414Y.0000000130
Down, J.L., MacDonald, M.A., Tétreault, J. & Williams, R.S. 1996. Adhesive testing at the Canadian Conservation Institute- an evaluation of selected poly(vinyl acetate) and acrylic adhesives. Stud Conserv. 41 (1): 19-44. https://doi.org/10.1179/sic.1996.41.1.19
Down J.L., Guild, S., Hill, G., St-Jacques, D., Westbury, K., O’Loughlin, E., Kaminska, E., Williams, R.S., Iraci, J. & Tse, S. 2011. Update on the CCI adhesive tape and heat-set tissues project. Pp. 57-84 in: Adhesives and consolidants for conservation : research and applications : proceedings of Symposium 2011. Adhésifs et consolidants pour la conservation : recherche et applications : actes du Symposium 2011. Ottawa: Canadian Conservation Institute.
Dupont, A.-L., Tétreault, J. & Tetreault, J. 2000. Cellulose degradation in an acetic acid environment. Stud Conserv. 45 (3): 201-210. https://doi.org/10.2307/1506766
Egenberg, I.M. & Moe, D. 1991. A "stop-press" announcement. Damage caused by a widely used herbarium mounting technique. Taxon 40 (4): 601-604. https://doi.org/10.2307/1222768
Fassett, N. 1952. Uses of cellulose acetate in the herbarium. Rhodora 54 (647): 286-288.
Feller, R.L. & Wilt, M. 1990. Evaluation of cellulose ethers for conservation. California: The Getty Conservation Institute.
Fuchs, R. 2008. Glues in paper restoration- new insights for preparation and application. Pp. 235-248 in: Fellows-Jensen, G. & Springborg, P. (eds.), Care and conservation of manuscripts 10, Proceedings of the tenth international seminar held at the University of Copenhagen, 19th-20th October 2006. Copenhagen: Museum Tusculanum Press, University of Copenhagen.
Gates, B. 1958. A new soil-binder for preserving lichen specimens. The Bryologist 61 (3): 249-252. https://doi.org/10.2307/3240475
Geiger, T., & Michel, F. 2005. Studies on the polysaccharide JunFunori used to consolidate matt paint. Stud Conserv. 50 (3): 193-204. https://doi.org/10.1179/sic.2005.50.3.193
Gersten, T., Michineau, M., Paternotre, A. & Thys, N. 2017. Herbarii Bruxellenses, le projet de conservation des herbiers de la Bibliothèque Royale de Belgique. In Monte Artium 10: 83-101. https://doi.org/10.1484/J.IMA.5.114683
Green, D. & Thickett, L.R. 1995. Testing materials for use in the storage and display of antiquities—a revised methodology, Stud Conserv, 40 (3): 145-152. https://doi.org/10.1179/sic.1995.40.3.145
Grenda, M. 2013. Ethical considerations concerning the restoration of a herbarium from the 19th century. Pp. 93-96 in: ICOM-CC Graphic Documents Working Group Interim Meeting | Vienna 17 – 19 April 2013. Vienna: ICOM-CC.
Grenda, M. 2015. Remedial conservation of a severely deteriorated 19th century bound herbarium. JoNSC 2: 54-59.
Grenda-Kurmanow, M. 2017. Conservation versus genetics. Challenges of conservation planning for historic herbaria in J. Bridgland (ed.), ICOM-CC 18th Triennial Conference Preprints, Copenhagen, 4-8 September 2017, art. 1103. Paris: International Council of Museums.
Grenda-Kurmanow, M. 2020, Adhesives used in herbaria: Current practice with regard to what we know from written sources on mounting herbarium specimens and conservation, Taxon, https://doi.org/10.1002/tax.12413
Gunn, A. 1994. Past and current practice: the botanist view. Pp. 11-14 in: Child, R.E. (ed.), Conservation and the herbarium. Leigh: The Institute of Paper Conservation.
Gutaker, R., Reiter, E., Furtwängler, A., Schuenemann, V.J. & Burbano, H.A., Extraction of ultrashort DNA molecules from herbarium specimens. BioTechniques. 62:76-79. https://doi.org/ 10.2144/000114517
Horie, V. 2010. Materials for conservation. Organic consolidants, adhesives, and coatings. 2nd ed. Pp. 5-8. London: Elsevier.
Jacobi E. & Phan Tan Luu, C. 2012. Herbaria restauratie-documentatie. Fase I. Conservation report, unpublished. Leiden: Nationaal Herbarium Nederland, Naturalis Biodiversity Center.
Keijzers, V. 2013. Historische herbaria: inzicht in de conservatie problematiek en een aanzet tot een leidraad voor de conservering en restauratie van gedroogd plantenmateriaal. MA thesis, Artesis Hogeschool Antwerpen: Artesis Hogeschool.
Kite, M. & Thomson, R. 2006. Conservation of leather and related materials. Oxford: Butterworth-Heinemann.
Lecky, M. 1972. Hortus Siccus. Gaevmans (Antonio) Volume I. Property of University of California, Los Angeles. Conservation report, unpublished. Leiden: Nationaal Herbarium Nederland, Naturalis Biodiversity Center.
Linnaeus, C. 1751. Philosophia Botanica in qua explicantur fundamenta botanica cum definitionibus partium, exemplis terminorum, observationibus rariorum adjectis figuris aeneis. Stockholm: Godofr. Kiesewetter. http://linnean-online.org/120027/
Lughandha, E. N., Walker, B. E., Canteiro, C., Chadburn, H., Davis, A.P., Hargreaves, S., Lucas, E.J., Schuiteman, A., Williams, E., Bachman, S.P., Baines, D., Amy Barker, Budden, A.P., Carretero, J., Clarkson, J.J., Roberts, A. & Rivers, M.C. 2019. The use and misuse of herbarium specimens in evaluating plant extinction risks. Phil.Trans. R. Soc. B 374 : 20170402. http://dx.doi.org/10.1098/rstb.2017.0402
Margez M. 2004. L'herbier Haller du Muséum national d'Histoire naturelle : un objet d'intérêt historique et scientifique. Thesis. Paris: Institut national du patrimoine. Departement des restaurateurs.
Menei E. 2005. Conservation et restauration d’herbiers : un cas exemplaire à la Bibliothèque de l'Institut de France. http://www.livre-franchecomte.com/download.cgi?filename=accounts/mnesys_accolad/datas/cms/Menei.pdf (accessed 27 May, 2020)
Merrill, E. 1926. A efficient and economical herbarium paste. Torreya 26 (4): 63-65.
Merrill, E. & Wyman, D. 1939. Index Kewensis in improved loose leafledger form. Bulletin of Popular Information (Arnold Arboretum, Harvard University) 7(8): 37-40.
Mertens, S. & Vries, de, H. 2013. Herbaria restauratie-documentatie. Fase II. Conservation report, unpublished. Leiden: Nationaal Herbarium Nederland, Naturalis Biodiversity Center.
Michel, F. 2011. Funori and JunFunori: Two Related Consolidants with Surprising Properties. Pp. 285-298 in: Adhesives and consolidants for conservation : research and applications : proceedings of Symposium 2011. Adhésifs et consolidants pour la conservation : recherche et applications : actes du Symposium 2011. Ottawa: Canadian Conservation Institute.
Nasatto, P.L., Pignon, F., Silveira, J.L.M., Duarte, M.E.R., Noseda, M.D. & Rinaudo, M. 2015. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 7: 777-803. https://doi.org/10.3390/polym7050777 Nesbitt, M. 2014. Chapter 22. Use of herbarium specimens in ethnobotany. Pp. 313-328 in: Salick, J., Konchar, K., Nesbitt, M. (eds.) Curating biocultural collections: a handbook. Kew: Kew Publishing
Phelan, H., Baer, N.S. & Indictor, N. 1971. An evaluation of adhesives for use in paper conservation. International Institute for Conservation of Historic and Artistic Works 11 (2): 58-75. https://doi.org/10.1179/019713671806030017
Poncy, O., Aupic, C. & Flament, G. 2012. La rénovation de l’Herbier du muséum national d’Histoire Naturelle. Coré 27: 1-10.
Popławska S. 2014a. Gdy suszone rośliny stają się zabytkiem. Historia, technologia, problem konserwacji i przechowywania zielników. MA thesis. Warszawa: Akademia Sztuk Pięknych w Warszawie.
Popławska S. 2014b. Problematyka konserwacji artystycznego zielnika i papeterii autorstwa Elizy Orzeszkowej podarowanych Leopoldowi Méyetowi, przechowywanych w Muzeum Narodowym w Warszawie. MA thesis. Warszawa: Akademia Sztuk Pięknych w Warszawie.
Rollins, R. 1955. The Archer method for mounting herbarium specimens. Rhodora 57 (682): 294-299.
Rytz, W. 1933, Das Herbarium Felix Platters. Ein Beitrag zur Geschichte der Botanik des XVI. Jahrhunderts. Band XLIV, 1. Teil. Basel: Separatabdruck aus den Verhandlungen der Naturforschenden Gesselschaft in Basel.
Schellmann, N. C. 2007. Animal glues: a review of their key properties relevant to conservation. Stud Conserv. 52 (sup1): 55-66. https://doi.org/10.1179/sic.2007.52.Supplement-1.55
Scheper, K. 2005. Een onderzoek naar Evacon-R. Gedrag en toepassingen van een witte lijm. CR: interdisciplinair vakblad voor conservering en restauratie 6 (1): 32-34. Sharp, A. J. 1964. A new method for mounting evergreen conifer twigs with deciduous leaves. Rhodora 66 (765): 216.
Shchepanek, M. 2001. In defence of using adhesives and low-temperature pest control for botanical specimens. Taxon 50 (1): 169-173. https://doi.org/10.2307/1224517
Smith, A. 2010. Herbaria and entomology preservation course. 18th-21st October 2010. Institut National du Patrimoine, Paris. NatSca News 21: 17-23.
Spiegel, van den, A. 1606. Isagoge in rem herbariam libri duo. Pp. 80-81. Padua: Paulus Meiettus, https://archive.org/details/BIUSante_pharma_res012063/mode/2up
Staats, M., Cuenca, A., Richardson, J.E., Vrielink-van Ginkel, R., Petersen, G., Seberg, O. & Bakker, F.T. 2011. DNA Damage in Plant Herbarium Tissue. PLoS One 6 (12): e28448. https://doi.org/:10.1371/journal.pone.0028448
Stevens, R., Garside, P. & Russell, E. 2011. A review of current and recent practice in the use of adhesives by the conservation department at the British Library. Pp. 28-40 in: Adhesives and consolidants for conservation: research and applications: proceedings of Symposium 2011. Adhésifs et consolidants pour la conservation: recherche et applications: actes du Symposium 2011. Ottawa: Canadian Conservation Institute.
Thiers, B. 2020. Index Herbariorum A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/science/ih/ (accessed 20 July, 2020)
Tillet, S. S. 1979. Technical aids for systematic botany II: New adhesives. Taxon 28 (4): 383-384. https://doi.org/10.2307/1219751
Tillet, S. S. 1989. Technical aids for systematic botany III. More ideas on methyl-cellulose adhesives. Taxon 38 (4): 597-601. https://doi.org/10.2307/1222635
Tournefort, de, J.P. 1694. Élémens de botanique: ou méthode pour connoître les plantes. Volume 1. Pp. 547-548. Paris: L'Imprimerie Royale. https://gallica.bnf.fr/ark:/12148/btv1b8454361d
Valk, de, M. 2010. Het Herbarium van de Stedelijke Musea Zierikzee. Beschrijving en conserveringsadvies. Conservation report, unpublished. Leiden: Nationaal Herbarium Nederland, Naturalis Biodiversity Center.
Valmont de Bomare, J.-C. 1775. Dictionnaire raisonné universel d'histoire naturelle, contenant l'histoire des animaux, des végétaux et des minéraux, et celle des corps célestes, des météores et autres principaux phénomènes de la nature. Pp. 582-584. T. IV. Paris: Brunet. https://gallica.bnf.fr/ark:/12148/bpt6k6433756q
Vries, de, H. 2013. Herbaria restauratie-documentatie. Fase II. Nationaal Herbarium Nederland. Conservation report, unpublished. Leiden: Nationaal Herbarium Nederland, Naturalis Biodiversity Center.
Walker, N. & Hughes, D. 1994. Conservation and the herbarium: the Royle Herbarium- a conservation approach. Pp. 35-41 in: Child, R.E. (ed.), Conservation and the Herbarium. Leigh: The Institute of Paper Conservation.
Weiss, C.L., Schuenemann, V.J., Devos, J., Shirsekar, G., Reiter, E., Gould, B.A., Stinchcombe, J.R., Krause, J. & Burbano, H.A. 2016. Temporal patterns of damage and decay kinetics of DNA retrieved from plant herbarium specimens. R. Soc. open sci. 3: 160239. http://dx.doi.org/10.1098/rsos.160239
Wierda, A. 1994. Het gebruik van o.a. PVA-lijm ter bevestiging van gedroogd plantenmateriaal in herbaria. Een onderzoeksrapport. Met een kort overzicht van bevestiginingsmethoden van plantenmateriaal op papier. Research report, unpublished. Den Haag: Algemeen Rijskarchief.
Wierda, A. 1995. De restauratie van het Boerhave Herbarium (Rijksherbarium Leiden), Amsterdam. Conservation report, unpublished. Leiden: Nationaal Herbarium Nederland, Naturalis Biodiversity Center.
Yesilyurt, J. 2009. Botanical related adhesives. NatSCA News 16: 30-31.
Yoo, Y. J. & Um, I. C. 2013. Examination of thermo-gelation behavior of HPMC and HEMC aqueous solutions using rheology. Korea-Aust Rheol J 25(2): 67-75. https://doi.org/10.1007/s13367-013-0007-8