Difference between revisions of "Fluid Collection Monitoring"
EmilyBraker (Talk | contribs) (→Topping up Tools) |
EmilyBraker (Talk | contribs) |
||
| (5 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Statement of Purpose == | == Statement of Purpose == | ||
| − | These links and documents contain information about best practices for monitoring fluid collections in natural history collections preserved in ethanol ( | + | These links and documents contain information about best practices for monitoring fluid collections in natural history collections preserved in ethanol (EtOH). For additional information on fluid collection curation and management, see the [[Fluid Collections]] page. |
==Introduction== | ==Introduction== | ||
| − | + | Fluid collections should be monitored on a regular schedule to prevent excessive preservative evaporation, detect faulty seals on storage containers or changes in preservative quality, and maintain collections at desired concentration levels. Monitoring intervals will vary based on staff resources as well as the environmental stability within a particular collection store; however, a general guideline is to monitor fluid collections every six months. | |
| − | Fluid collections should be monitored on a regular schedule to prevent excessive preservative evaporation, detect faulty seals on storage containers or changes in preservative quality, and | + | |
==Preventing Evaporation== | ==Preventing Evaporation== | ||
| − | |||
Preservative evaporation within containers steadily occurs in the collection environment. Several measures can be implemented to slow evaporation rates, such as buffering the collection space from fluctuations in temperature and humidity through use of environmental controls, and placement of the store within an interior space rather than adjacent to exterior walls that are more vulnerable to climactic variations. Use of best practice housing materials can also reduce within-jar evaporation rates. Polypropylene lids are less prone to embrittlement than harder plastics such as Bakelite, and can be lined with a polyethylene or Teflon insert to act as a mild oxygen barrier. Use of a film layer (e.g., Teflon, Parafilm) between lid and container or Telfon tape wrapped along the threading on jar mouths can also slow evaporation rates, <ref>Simmons, John E. 2002. Herpetological Collecting and Collections Management. Rev. ed. Society for the Study of Amphibians and Reptiles Herpetological Circular 3. New York: Taylor and Francis.</ref> especially when best practice materials are unavailable. Although common, rubber gaskets used with wire-bail jars are not recommended due to the fact that they may dissolve or embrittle when coming into contact with ethanol, risking contamination of container contents in addition to negating the efficacy of the seal. | Preservative evaporation within containers steadily occurs in the collection environment. Several measures can be implemented to slow evaporation rates, such as buffering the collection space from fluctuations in temperature and humidity through use of environmental controls, and placement of the store within an interior space rather than adjacent to exterior walls that are more vulnerable to climactic variations. Use of best practice housing materials can also reduce within-jar evaporation rates. Polypropylene lids are less prone to embrittlement than harder plastics such as Bakelite, and can be lined with a polyethylene or Teflon insert to act as a mild oxygen barrier. Use of a film layer (e.g., Teflon, Parafilm) between lid and container or Telfon tape wrapped along the threading on jar mouths can also slow evaporation rates, <ref>Simmons, John E. 2002. Herpetological Collecting and Collections Management. Rev. ed. Society for the Study of Amphibians and Reptiles Herpetological Circular 3. New York: Taylor and Francis.</ref> especially when best practice materials are unavailable. Although common, rubber gaskets used with wire-bail jars are not recommended due to the fact that they may dissolve or embrittle when coming into contact with ethanol, risking contamination of container contents in addition to negating the efficacy of the seal. | ||
===Risks of Evaporation=== | ===Risks of Evaporation=== | ||
| − | Mixed together, ethanol and water form an azeotrope rather than a true solution,<ref>Simmons, John E. 2014. Fluid Preservation: A Comprehensive Reference. Lanham, MD: Rowman & Littlefield, 347 pp. </ref> resulting in asymmetric rates of evaporation. Ethanol evaporates faster from mixture than water, diluting preservative strength over time. When concentrations fall below desired levels, specimens are at risk of degradation. Ethanol’s preservative qualities diminish at concentrations <60%, and specimen cell autolysis, decomposition and deterioration may occur. <ref>Notton David G. 2010. Maintaining concentration: a new practical method for profiling and topping up alcohol-preserved collections. Collection Forum, 24, 1-27. </ref> At low concentration strengths (<50%), ethanol loses its antiseptic properties, promoting bacterial and fungal growth. <ref>Waller, R. and T.J.K. Strang. 1996. Physical chemical properties of preservative solutions—I. Ethanol–water solutions. Collection Forum 12(2):70–85.</ref> Specimens exposed to air from dropping preservative levels are vulnerable to desiccation, salt and solute deposition, and increased risk of mechanical injury. | + | Mixed together, ethanol and water form an azeotrope rather than a true solution,<ref>Simmons, John E. 2014. Fluid Preservation: A Comprehensive Reference. Lanham, MD: Rowman & Littlefield, 347 pp. </ref> resulting in asymmetric rates of evaporation. Ethanol evaporates faster from mixture than water, diluting preservative strength over time. When concentrations fall below desired levels, specimens are at risk of degradation. Ethanol’s preservative qualities diminish at concentrations <60%, and specimen cell autolysis, decomposition, and deterioration may occur. <ref>Notton David G. 2010. Maintaining concentration: a new practical method for profiling and topping up alcohol-preserved collections. Collection Forum, 24, 1-27. </ref> At low concentration strengths (<50%), ethanol loses its antiseptic properties, promoting bacterial and fungal growth. <ref>Waller, R. and T.J.K. Strang. 1996. Physical chemical properties of preservative solutions—I. Ethanol–water solutions. Collection Forum 12(2):70–85.</ref> Specimens exposed to air from dropping preservative levels are vulnerable to desiccation, salt and solute deposition, and increased risk of mechanical injury. |
==Topping Up== | ==Topping Up== | ||
| Line 20: | Line 18: | ||
Preservative requirements vary by collection type. Most vertebrate collections are maintained at 70% ethanol while invertebrate collections have a target range of 75-85% ETOH concentration depending on whether or not organisms are soft-bodied. Achieving a desirable preservative strength is a balancing act – concentrations must be strong enough to serve as an effective biocide (>50% ETOH) yet minimize dehydration to specimens, which experience warping and shrinkage at high concentrations (>80% ETOH). | Preservative requirements vary by collection type. Most vertebrate collections are maintained at 70% ethanol while invertebrate collections have a target range of 75-85% ETOH concentration depending on whether or not organisms are soft-bodied. Achieving a desirable preservative strength is a balancing act – concentrations must be strong enough to serve as an effective biocide (>50% ETOH) yet minimize dehydration to specimens, which experience warping and shrinkage at high concentrations (>80% ETOH). | ||
| − | When fluid levels have noticeably dropped inside jars, it is necessary to add more preservative, or “top up” | + | When fluid levels have noticeably dropped inside jars, it is necessary to add more preservative, or “top up” to maintain the desired concentration of the preservative and ensure that all parts of a specimen remain submerged in the preservative. It is common practice for collections to routinely top-up through loose approximation of the preservative strength needed to return containers to target concentration, either by adding storage-grade preservative (e.g., 70% ETOH) or by adding full-strength preservative (e.g., 95% ETOH) in an attempt to counteract any evaporation that has already occurred. Both of these approaches have their drawbacks. For instance, the addition of storage-grade preservative does not account for evaporation that has already occurred in the container, and no amount of storage-grade preservative will return a jar that has experienced some degree of evaporation to desired strength, but will instead slowly dilute the solution over time. Conversely, adding full-strength preservative without knowledge of the starting concentration can either over-concentrate the storage solution (causing specimen dehydration, distortion and shrinkage), or fail to bring the solution to the target concentration in the case of extremely diluted preservative. |
| − | + | The best practice <ref>Notton David G. 2010. Maintaining concentration: a new practical method for profiling and topping up alcohol-preserved collections. Collection Forum, 24, 1-27. </ref> approach to topping up is to measure: | |
| + | # the starting concentration of the preservative and | ||
| + | # the initial volume of the preservative. | ||
| + | Together, these metrics will indicate the correct concentration of preservative necessary to bring the solution to desired concentration when filling the container. Initial volume is a necessary data point for topping up as it conveys the physical space available within which to increase preservative strength. In the case of highly diluted solutions, it is often necessary to pour off some of the existing preservative to create sufficient space for the amount of full-strength preservative needed to bring he solution up to the target concentration. See [[#ref6|Notton 2010]] for greater detail on this method and a summary protocol in the [https://spnhc.biowikifarm.net/w/index.php?title=File:Topping_Up_Protocol_-_Calculator.pdf&page=2 downloadable tool] provided. | ||
===Quantitative Topping up Tools=== | ===Quantitative Topping up Tools=== | ||
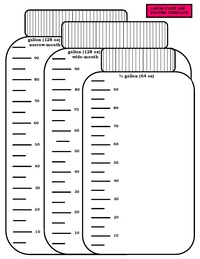
| − | A hydrometer or digital density meter is needed to accurately measure initial preservative concentration while preservative volume can be measured using a dipstick or a [https://spnhc.biowikifarm.net/w/index.php?title=File:Jar_Templates.pdf&page=2 jar volume template]. From these metrics, the necessary concentration required to return a container to target preservative strength can be calculated. However, individual calculations can be time-consuming when topping up a large collection. Instead, a reference table format is desirable so that technicians can quickly | + | A hydrometer or digital density meter is needed to accurately measure initial preservative concentration while preservative volume can be measured using a dipstick or a [https://spnhc.biowikifarm.net/w/index.php?title=File:Jar_Templates.pdf&page=2 jar volume template]. From these metrics, the necessary concentration required to return a container to target preservative strength can be calculated. However, individual calculations can be time-consuming when topping up a large collection. Instead, a reference table format is desirable so that technicians can quickly and accurately determine the concentration to be added to jars based on approximations of volume and concentration of the container at hand. A tool that makes these calculations was developed by [[#ref6|Notton 2010]] for invertebrate collections, with a version adapted for vertebrate collections available for [https://spnhc.biowikifarm.net/w/index.php?title=File:Topping_Up_Protocol_-_Calculator.pdf&page=2 download] that provides step-by-step instructions. |
==Future Monitoring== | ==Future Monitoring== | ||
| Line 35: | Line 36: | ||
[[User:EmilyBraker|Emily Braker]] | [[User:EmilyBraker|Emily Braker]] | ||
| − | == | + | ==Additional Resources== |
| − | + | ||
[http://www.spnhc.org/media/assets/cofo_2009_V23N12.pdf Cato, P.S. 1990. Characteristics of a collection of fluid-preserved mammals and implications for collections management. Collection Forum 6(2):53–64.] | [http://www.spnhc.org/media/assets/cofo_2009_V23N12.pdf Cato, P.S. 1990. Characteristics of a collection of fluid-preserved mammals and implications for collections management. Collection Forum 6(2):53–64.] | ||
| Line 50: | Line 50: | ||
[http://www.spnhc.org/media/assets/cofo_1996_V12N2.pdf Waller, R. and T.J.K. Strang. 1996. Physical chemical properties of preservative solutions—I. Ethanol–water solutions. Collection Forum 12(2):70–85.] | [http://www.spnhc.org/media/assets/cofo_1996_V12N2.pdf Waller, R. and T.J.K. Strang. 1996. Physical chemical properties of preservative solutions—I. Ethanol–water solutions. Collection Forum 12(2):70–85.] | ||
| − | [[Category: | + | |
| + | ==References== | ||
| + | <references/> | ||
| + | |||
| + | [[Category: Best Practices]][[Category:Zoology Collections]][[Category:Botanical Collections]][[Category:Specimen and Material Type]][[Category:Curation Practices]] | ||
Latest revision as of 18:05, 14 December 2020
Contents
Statement of Purpose
These links and documents contain information about best practices for monitoring fluid collections in natural history collections preserved in ethanol (EtOH). For additional information on fluid collection curation and management, see the Fluid Collections page.
Introduction
Fluid collections should be monitored on a regular schedule to prevent excessive preservative evaporation, detect faulty seals on storage containers or changes in preservative quality, and maintain collections at desired concentration levels. Monitoring intervals will vary based on staff resources as well as the environmental stability within a particular collection store; however, a general guideline is to monitor fluid collections every six months.
Preventing Evaporation
Preservative evaporation within containers steadily occurs in the collection environment. Several measures can be implemented to slow evaporation rates, such as buffering the collection space from fluctuations in temperature and humidity through use of environmental controls, and placement of the store within an interior space rather than adjacent to exterior walls that are more vulnerable to climactic variations. Use of best practice housing materials can also reduce within-jar evaporation rates. Polypropylene lids are less prone to embrittlement than harder plastics such as Bakelite, and can be lined with a polyethylene or Teflon insert to act as a mild oxygen barrier. Use of a film layer (e.g., Teflon, Parafilm) between lid and container or Telfon tape wrapped along the threading on jar mouths can also slow evaporation rates, [1] especially when best practice materials are unavailable. Although common, rubber gaskets used with wire-bail jars are not recommended due to the fact that they may dissolve or embrittle when coming into contact with ethanol, risking contamination of container contents in addition to negating the efficacy of the seal.
Risks of Evaporation
Mixed together, ethanol and water form an azeotrope rather than a true solution,[2] resulting in asymmetric rates of evaporation. Ethanol evaporates faster from mixture than water, diluting preservative strength over time. When concentrations fall below desired levels, specimens are at risk of degradation. Ethanol’s preservative qualities diminish at concentrations <60%, and specimen cell autolysis, decomposition, and deterioration may occur. [3] At low concentration strengths (<50%), ethanol loses its antiseptic properties, promoting bacterial and fungal growth. [4] Specimens exposed to air from dropping preservative levels are vulnerable to desiccation, salt and solute deposition, and increased risk of mechanical injury.
Topping Up
Preservative requirements vary by collection type. Most vertebrate collections are maintained at 70% ethanol while invertebrate collections have a target range of 75-85% ETOH concentration depending on whether or not organisms are soft-bodied. Achieving a desirable preservative strength is a balancing act – concentrations must be strong enough to serve as an effective biocide (>50% ETOH) yet minimize dehydration to specimens, which experience warping and shrinkage at high concentrations (>80% ETOH).
When fluid levels have noticeably dropped inside jars, it is necessary to add more preservative, or “top up” to maintain the desired concentration of the preservative and ensure that all parts of a specimen remain submerged in the preservative. It is common practice for collections to routinely top-up through loose approximation of the preservative strength needed to return containers to target concentration, either by adding storage-grade preservative (e.g., 70% ETOH) or by adding full-strength preservative (e.g., 95% ETOH) in an attempt to counteract any evaporation that has already occurred. Both of these approaches have their drawbacks. For instance, the addition of storage-grade preservative does not account for evaporation that has already occurred in the container, and no amount of storage-grade preservative will return a jar that has experienced some degree of evaporation to desired strength, but will instead slowly dilute the solution over time. Conversely, adding full-strength preservative without knowledge of the starting concentration can either over-concentrate the storage solution (causing specimen dehydration, distortion and shrinkage), or fail to bring the solution to the target concentration in the case of extremely diluted preservative.
The best practice [5] approach to topping up is to measure:
- the starting concentration of the preservative and
- the initial volume of the preservative.
Together, these metrics will indicate the correct concentration of preservative necessary to bring the solution to desired concentration when filling the container. Initial volume is a necessary data point for topping up as it conveys the physical space available within which to increase preservative strength. In the case of highly diluted solutions, it is often necessary to pour off some of the existing preservative to create sufficient space for the amount of full-strength preservative needed to bring he solution up to the target concentration. See Notton 2010 for greater detail on this method and a summary protocol in the downloadable tool provided.
Quantitative Topping up Tools
A hydrometer or digital density meter is needed to accurately measure initial preservative concentration while preservative volume can be measured using a dipstick or a jar volume template. From these metrics, the necessary concentration required to return a container to target preservative strength can be calculated. However, individual calculations can be time-consuming when topping up a large collection. Instead, a reference table format is desirable so that technicians can quickly and accurately determine the concentration to be added to jars based on approximations of volume and concentration of the container at hand. A tool that makes these calculations was developed by Notton 2010 for invertebrate collections, with a version adapted for vertebrate collections available for download that provides step-by-step instructions.
Future Monitoring
Following remedial topping up using the quantitative approach described above, a shorthand monitoring system can be implemented for several monitoring cycles given that variation in concentration should be minimal once all containers are accurately reset to target concentration (assuming seals are effective and environmental conditions in the collection store are relatively stable). If all containers are filled to the same benchmark (e.g., neck of jar), a drop in volume is easily detected and quantified based on the divergence from the start level. General rules based on average evaporation rates particular to a store can be implemented (e.g., if volume reduced by ½ inch, add 80% ETOH, if volume reduced by >10% replace seal). After 4-5 shorthand topping up cycles, full monitoring using a hydrometer/density meter and topping up tool should be applied. If episodic monitoring protocols are maintained, this full monitoring protocol should be less intensive than the initial remediation topping up activity.
Contributors
Additional Resources
Conservation and Collections Care. 2016. Standards in the Care of Wet Care of Collections. [online] Available at: http://conservation.myspecies.info/node/33
Simmons, John E. 2002. Herpetological Collecting and Collections Management. Rev. ed. Society for the Study of Amphibians and Reptiles Herpetological Circular 3. New York: Taylor and Francis.
Simmons, John E. 2014. Fluid Preservation: A Comprehensive Reference. Lanham, MD: Rowman & Littlefield, 347 pp.
References
- ↑ Simmons, John E. 2002. Herpetological Collecting and Collections Management. Rev. ed. Society for the Study of Amphibians and Reptiles Herpetological Circular 3. New York: Taylor and Francis.
- ↑ Simmons, John E. 2014. Fluid Preservation: A Comprehensive Reference. Lanham, MD: Rowman & Littlefield, 347 pp.
- ↑ Notton David G. 2010. Maintaining concentration: a new practical method for profiling and topping up alcohol-preserved collections. Collection Forum, 24, 1-27.
- ↑ Waller, R. and T.J.K. Strang. 1996. Physical chemical properties of preservative solutions—I. Ethanol–water solutions. Collection Forum 12(2):70–85.
- ↑ Notton David G. 2010. Maintaining concentration: a new practical method for profiling and topping up alcohol-preserved collections. Collection Forum, 24, 1-27.